Method for preparing genetically engineered bacteria for efficiently compounding pantothenic acid and application thereof
A technology of genetically engineered bacteria and pantothenic acid, applied in the direction of microorganism-based methods, biochemical equipment and methods, bacteria, etc., can solve the problems of low enzyme activity, substrate toxicity, high production cost, etc., achieve high yield, short fermentation time, highly active effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Construction of Genetically Engineered Bacteria
[0019] Respectively selected from Escherichia coli (Escherichia coli BL21 (DE3)), Corynebacterium glutamicum ATCC 13032, Bacillus subtilis (Bacillus subtilissubsp.subtilis str.168), Bacillus cereus (Bacillus cereus E33L), Enterobacter cloacae EcWSU1, Bacillus thuringiensis BMB171, and Escherichia coli high-efficiency expression system BL21(DE3) / PET30 were used to construct genetically engineered bacteria.
[0020] Using Escherichia coli (E.coliBL21(DE3)) panc gene (Sequence Listing 1) as a template, the fragment panC was obtained by PCR amplification with primers E-panc-for and E-panc-rev respectively E , the PCR product panC E The fragment was recovered by 1% agarose electrophoresis, and after double digestion with restriction endonucleases BamH 1 and Xho 1, it was connected to the vector pET30 after the same digestion to obtain the recombinant plasmid pET30-panC E , transform E.coli DH5α competent cells, colony PCR s...
Embodiment 2
[0030] Enzyme Activity Detection of Genetic Engineering Strains
[0031] Pick up E.coli BL21(DE3) / pET30-panC from the plate E , E.coli BL21(DE3) / pET30-panC C , E.coli BL21(DE3) / pET30-panC b , E.coli BL21(DE3) / pET30-panC bc , E.coli BL21(DE3) / pET30-panC ec and E.coli BL21(DE3) / pET30-panC bt A single colony was inoculated in 5 mL of LB medium (containing 50 μg / ml Kan) and cultured overnight at 37°C. The above culture solution was transferred to 20 mL of LB medium (containing 50 μg / ml Kan) at a ratio of 1:100, cultured at 37°C, and when the OD reached 0.4-0.6, 0.2mM IPTG was added, and cultured at 30°C for 16h. Collect 10OD bacteria, use 1ml buffer (100mM Hepes, 20mM MgCl 2 .6H 2 O, 1 mM EDTA, pH 8.0) to suspend, then sonicate the bacterial cells, centrifuge to get the supernatant and dilute 10 times. Take 5 μL of the diluted liquid and add 1.1 mL of reaction buffer (25 mM β-alanine, 25 mM DL-pantoic acid, 4.5 mM ATP, 10 mM MgCl 2 , 15mM KCl). React at 37°C for 20 minut...
Embodiment 3
[0038] high density fermentation
[0039] (1) Pick the engineering bacteria E.coli BL21(DE3) / PET30-panC containing the pantothenate synthase of Corynebacterium glutamicum from the plate C Single colonies were inoculated in 10 mL of LB liquid medium ((yeast extract 5 g / L, peptone 10 g / L, sodium chloride 10 g / L)), and cultivated overnight at 37 °C;
[0040] (2) Take 3mL and transfer to 100mL high-density fermentation medium (glucose 20g / L, (NH 4 ) 2 SO 4 9g / L, Na 2 CO 3 2g / L, KH 2 PO 4 6.67g / L, (NH 4 ) 2 HPO 4 4g / L, MgSO 4 ·7H 2 O 0.8g / L, citric acid 0.8g / L, NaHCO 3 2g / L, 5mL ion mother solution, pH 7.0), 50μg / ml kanamycin (Kan), cultured at 37°C for 12h;
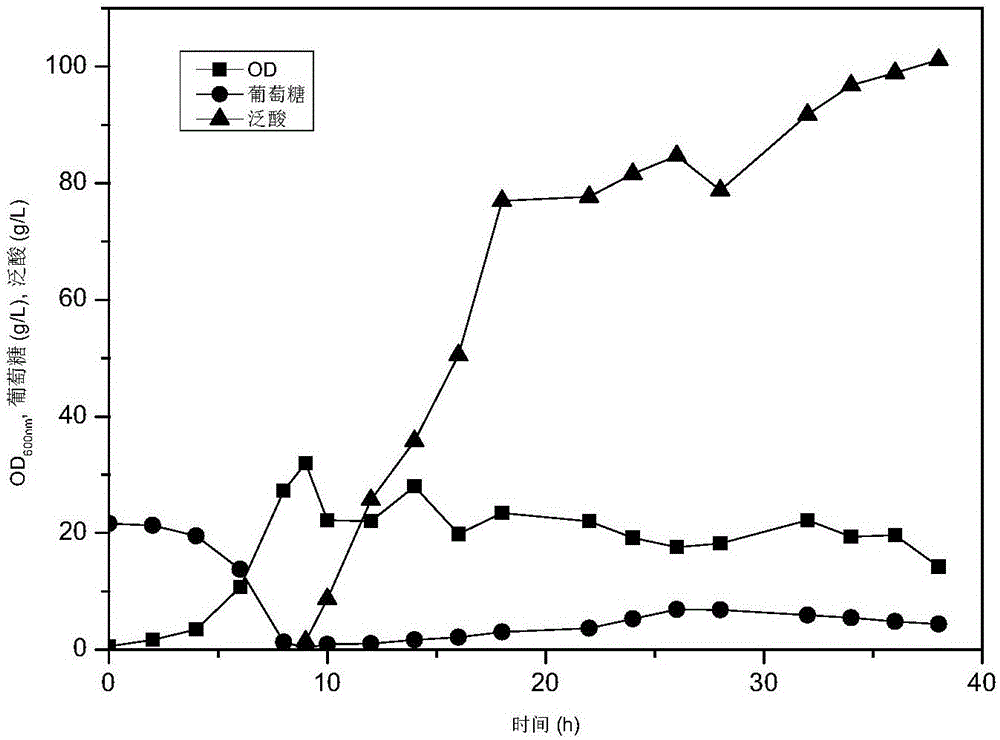
[0041] (3) Take 60ml of the 100ml medium in the second step, and transfer it to a 1L fermenter containing 600mL of fermentation medium at a ratio of 1:10 for cultivation, control dissolved oxygen at 15%, and cultivate at 30°C; ferment for 8h Add feeding material (glucose, 650g / L); Add IPTG (final concentratio...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com