Medicine composition and application thereof
A composition and drug technology, applied in drug combination, drug delivery, medical formula, etc., can solve problems such as uncertain sedative effect, easy to be awakened by external stimuli, pain stimulation, etc., to achieve strong controllability of waking up and drug onset Fast, less side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of sedative medicine, containing dexmedetomidine hydrochloride and ketamine hydrochloride and pharmaceutically acceptable auxiliary materials, it is prepared into nasal spray, and it is prepared according to the following steps:
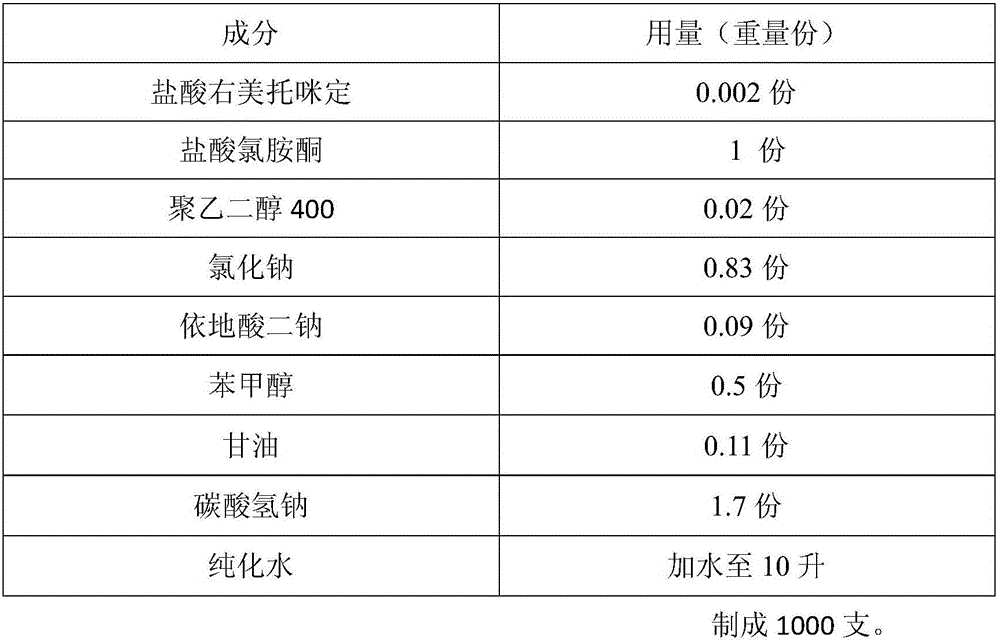
[0029] Prescription composition:
[0030]
[0031] 1. Take 2L of purified water and put it in the liquid mixing tank, set the mixing speed to 60-80 rpm, slowly add the prescribed amount of polyethylene glycol 400, stir while adding, after adding the polyethylene glycol 400, continue stir, spare;
[0032] 2. After continuing to stir for 20 minutes, add dexmedetomidine hydrochloride, ketamine hydrochloride, glycerin, sodium chloride, disodium edetate, benzyl alcohol in sequence, add purified water until the volume of the liquid is 8L, and continue stirring to dissolve ,spare;
[0033] 3. While stirring, add sodium bicarbonate to adjust the pH of the solution to 5.0-7.0, make up the remaining purified water, filter it with a 0.22 micr...
Embodiment 2
[0036] A kind of sedative medicine, containing dexmedetomidine hydrochloride and ketamine hydrochloride and pharmaceutically acceptable adjuvants, it is prepared into injection, according to the following steps:
[0037] Element Dosage (parts by weight) Dexmedetomidine Hydrochloride 0.005 copies Ketamine hydrochloride 0.5 parts Mannitol 3.7 servings Sodium chloride 1.1 copies Sterile water for injection Add to 2000ml
[0038] Preparation process:
[0039] 1. Concentrated formulation: Add the above-mentioned raw and auxiliary materials into the batching tank, then add 1 / 3 of the prescription amount of sterile water for injection, stir, dissolve, and obtain a concentrated formulation;
[0040] 2. Dilute preparation: take the concentrated preparation, add 0.1mol / L sodium bicarbonate solution, adjust the pH to 6.5-7.0, stir, mix well, filter with a 0.8μm filter membrane, and add 0.1%-0.3% mass of the total volume Activated carbon...
Embodiment 3
[0045] A kind of sedative medicine, containing dexmedetomidine hydrochloride and ketamine hydrochloride and pharmaceutically acceptable adjuvant, it is prepared into tablet, according to the following steps:
[0046]
[0047] Preparation process:
[0048] 1. Dexmedetomidine hydrochloride, ketamine hydrochloride, magnesium stearate, magnesium lauryl sulfate, sodium carboxymethyl starch and starch were pulverized respectively, passed through a 80-mesh sieve, and set aside;
[0049] 2. Mix dexmedetomidine hydrochloride, ketamine hydrochloride, magnesium lauryl sulfate, sodium carboxymethyl starch and starch evenly, and add 20% ethanol solution to make a soft material;
[0050] 3. Take the soft material obtained above, granulate it with a 18-mesh sieve, place the granules in a hot air oven, set the temperature at 60°C, dry until the moisture content of the granules is ≤3%, and granulate with an 18-mesh sieve;
[0051] 4. Take the above granules, add lubricant, and mix well;
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com