Total synthesis method of chloromycin A and analogue
A technique for griseogreenin and analogs, which is applied in the field of total synthesis of griseogreenin A and analogs, and achieves the effects of simple reaction steps, mild reaction conditions and wide adaptability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
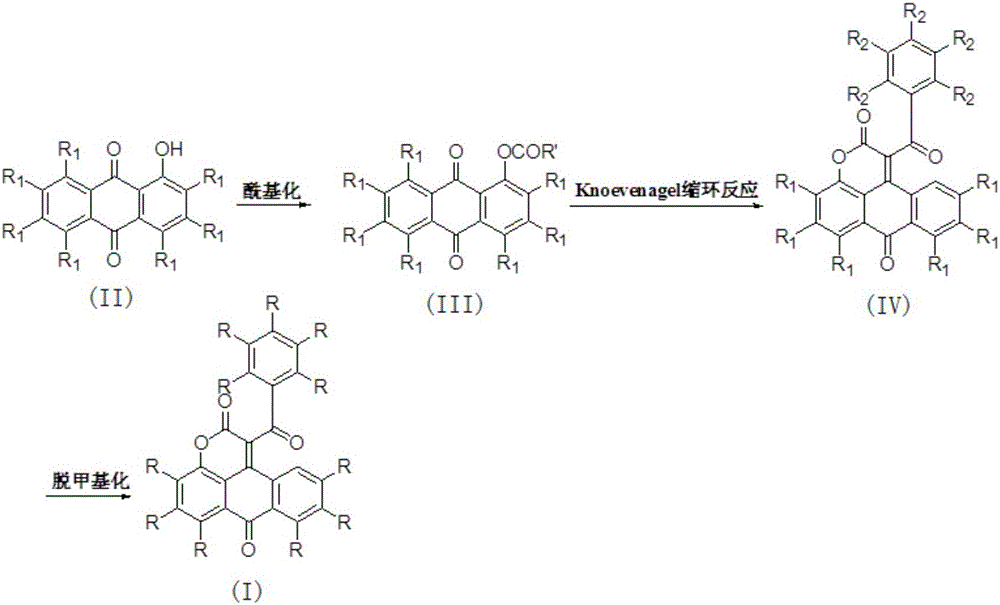
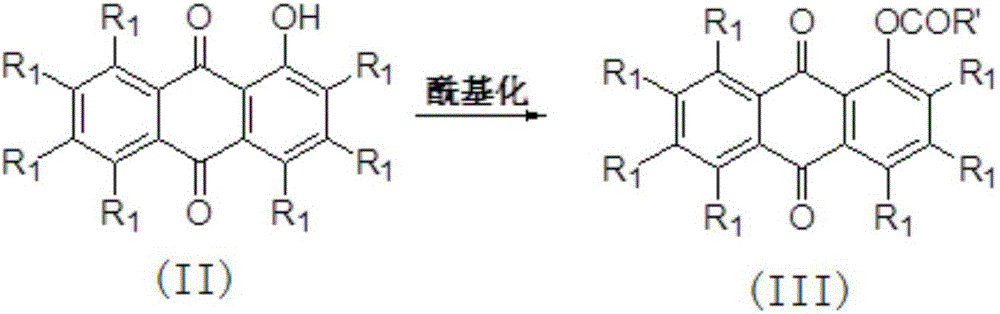
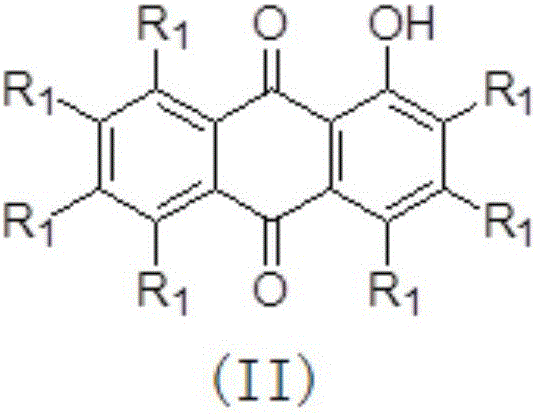
[0033] Example 1: Preparation of 4-methoxy-1-acetoxy-9,10-anthraquinone
[0034] 1-Hydroxy-4-methoxy-9,10-anthraquinone (2.0 g, 7.87 mmol) was dissolved in 100 mL of dichloromethane, and triethylamine (6.04 mL, 43.29 mmol) and diethyl Acyl chloride (2.23mL, 31.48mmol), raised to room temperature and continued stirring for 1 hour, concentrated under reduced pressure, extracted and filtered with dichloromethane, washed the extract with saturated brine, dried over anhydrous sodium sulfate, filtered, concentrated under reduced pressure, and the remaining The product was separated by flash column chromatography (V (petroleum ether (60~90°C): V (ethyl acetate) = 2:1, R f =0.275), to obtain 2.0 g of 4-methoxy-9,10-anthraquinone-1-acetate, with a yield of 86%. Orange-yellow solid, M.p.: 185°C, 1 HNMR (CDCl 3 ,400MHz,23℃),δ8.2(m,1H),8.1(m,1H),7.7(m,2H),7.3(m,2H),4.0(s,3H),2.5(s, 3H) ; MS (APCI) for C 17 h 12 o 5 (M+H + ): 297.1.
Embodiment 2
[0035] Example 2: Preparation of 4-methoxy-1-benzoyloxy-9,10-anthraquinone
[0036] Dissolve 1-hydroxy-4-methoxy-9,10-anthraquinone (2g, 7.87mmol) in 100mL of dichloromethane, add triethylamine (6.04mL, 43.3mmol) and benzoyl chloride successively under ice-cooling (3.63mL, 31.5mmol), after continuing to react at room temperature for 2 hours, washed with saturated brine, concentrated under reduced pressure, and the residue was separated by flash column chromatography (V (petroleum ether (60-90°C): V (ethyl acetate) = 2:1, R f =0.35), to obtain 2.45 g of orange-yellow solid 4-methoxy-1-benzoyloxy-9,10-anthraquinone with a yield of 87%. Orange-yellow solid, M.p.: 214°C, 1 H NMR (CDCl 3 ,400MHz,23℃), δ8.31(dd,2H),8.25(d,1H),8.11(d,1H),7.78(m,3H),7.61(m,2H),7.52(d,1H) ,7.45(d,1H),4.10(s,3H); MS(APCI) for C 22 h 14 o 6 (M+H + ): 358.9.
Embodiment 3
[0037] Example 3: Preparation of 6-methoxy-(1-benzoyl)naphtho[1,2,3-de]benzopyran-2,7-dione
[0038] Under nitrogen, anhydrous potassium carbonate (120 mg, 0.85 mmol), ethyl benzoylacetate (43 μL, 0.225 mmol), 1-acetic acid-4-methoxyanthraquinone ester (50 mg, 0.17 mmol) and DMSO ( 2.0 mL), after reacting at 40°C for 24 hours, add 10 mL of water, extract with dichloromethane (3×30 mL), combine the extracts, dry over anhydrous sodium sulfate, filter, concentrate under reduced pressure, and the residue is separated by flash column chromatography ( V (petroleum ether (60-90°C): V (ethyl acetate) = 2:1, Rf = 0.13), to obtain 6-methoxy-1-benzoylnaphthyl[1,2,3-de]benzene Pyran-2,7-dione 72 mg, yield 55%. Tan solid, M.p.: 224°C, 1 H NMR (CDCl 3 ,400MHz,23℃),δ8.47(dd,1H),8.07(d,2H),7.85(d,1H),7.73(m,3H),7.55(m,2H),7.47(m,2H) ,4.14(s,3H); MS(APCI) for C 24 h 14 o 5 (M+H + ): 383.1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com