A kind of green fluorescent ceramic material, preparation method and application thereof
A technology of green fluorescent and ceramic materials, applied in luminescent materials, chemical instruments and methods, light sources, etc., can solve the problems of lack of green and red components, poor color reproduction, and cold tones, etc., and achieve pure chroma and production conditions And the equipment requirements are not high, the effect of crystallinity is good
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of Ca 9.9 Tb 0.1 Si 3 o 15 f 2, according to the chemical formula Ca 9.9 Tb 0.1 Si 3 o 15 f 2 The stoichiometric ratio of each element in, weighed calcium carbonate CaCO 3 : 8.908 g, silicon oxide SiO 2 : 1.8024 g, terbium oxide Tb 2 o 3 : 0.0019 g, ground and mixed in an agate mortar, pre-sintered in an air atmosphere, the sintering temperature is 800 ° C, the sintering time is 10 hours, then cooled to room temperature, and the sample is taken out; after grinding and mixing again, the raw material is again mixed with 0.7808 g calcium fluoride CaF 2 Thoroughly mix and grind evenly, press the mixed powder into shape at a pressure of 10 MPa, and calcinate again in an air atmosphere at a calcining temperature of 1200°C for 5 hours, cool naturally, grind and mix evenly to obtain fluorescent ceramics.
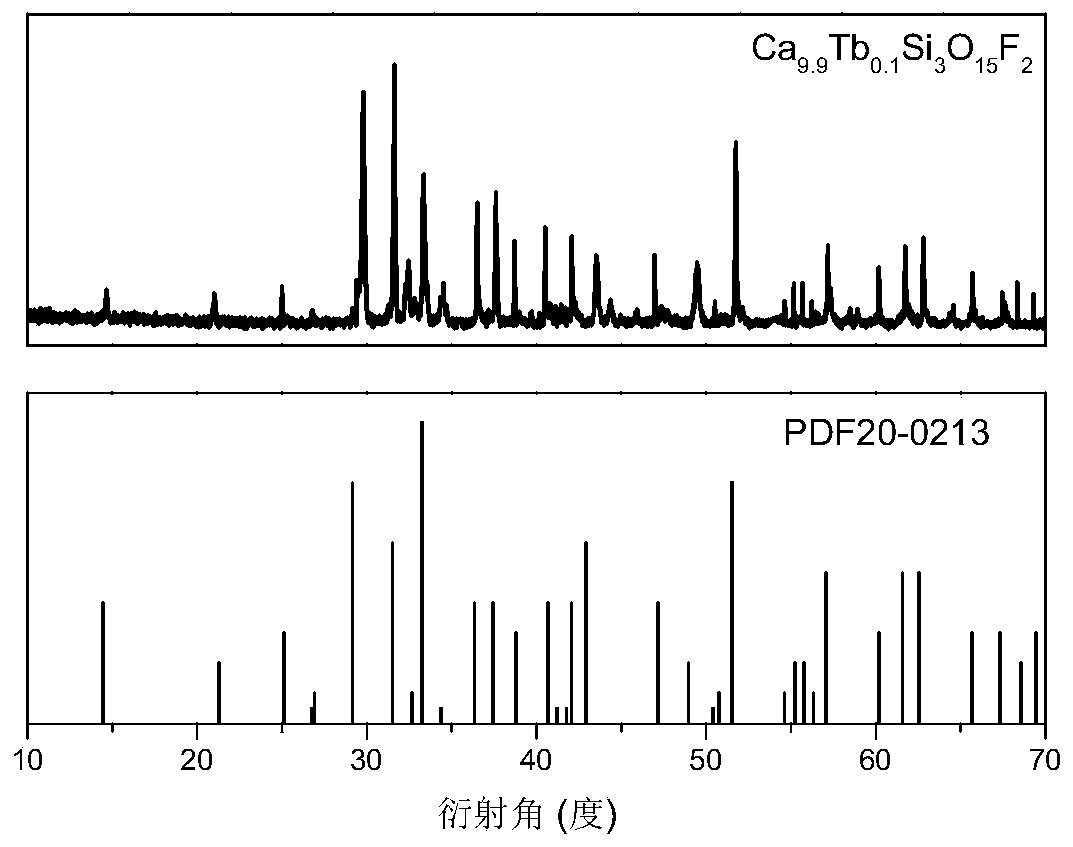
[0031] See attached figure 1 , which is the X-ray powder diffraction pattern of the sample prepared by the technical scheme of this embodiment, the ...
Embodiment 2
[0037] Preparation of Ca 9.99 Tb 0.01 Si 3 o 15 f 2 , according to the chemical formula Ca 9.9 Tb 0.1 Si 3 o 15 f 2 The stoichiometric ratio of each element in , respectively weigh calcium hydroxide Ca(OH) 2 : 5.1793 g, silicic acid H 2 SiO 3 : 2.34 g, terbium oxide Tb 2 o 3 : 0.00021 g was ground in an agate mortar and mixed evenly, and then pre-sintered in an air atmosphere. The sintering temperature was 750 ° C, and the sintering time was 10 hours. Then, it was cooled to room temperature, and the sample was taken out; g calcium fluoride CaF 2 Fully mix and grind evenly, press the mixed powder into shape, the pressure is 15MPa, and calcined again in air atmosphere, the calcining temperature is 1200°C, the calcining time is 3 hours, and the fluorescent ceramic material is obtained by natural cooling.
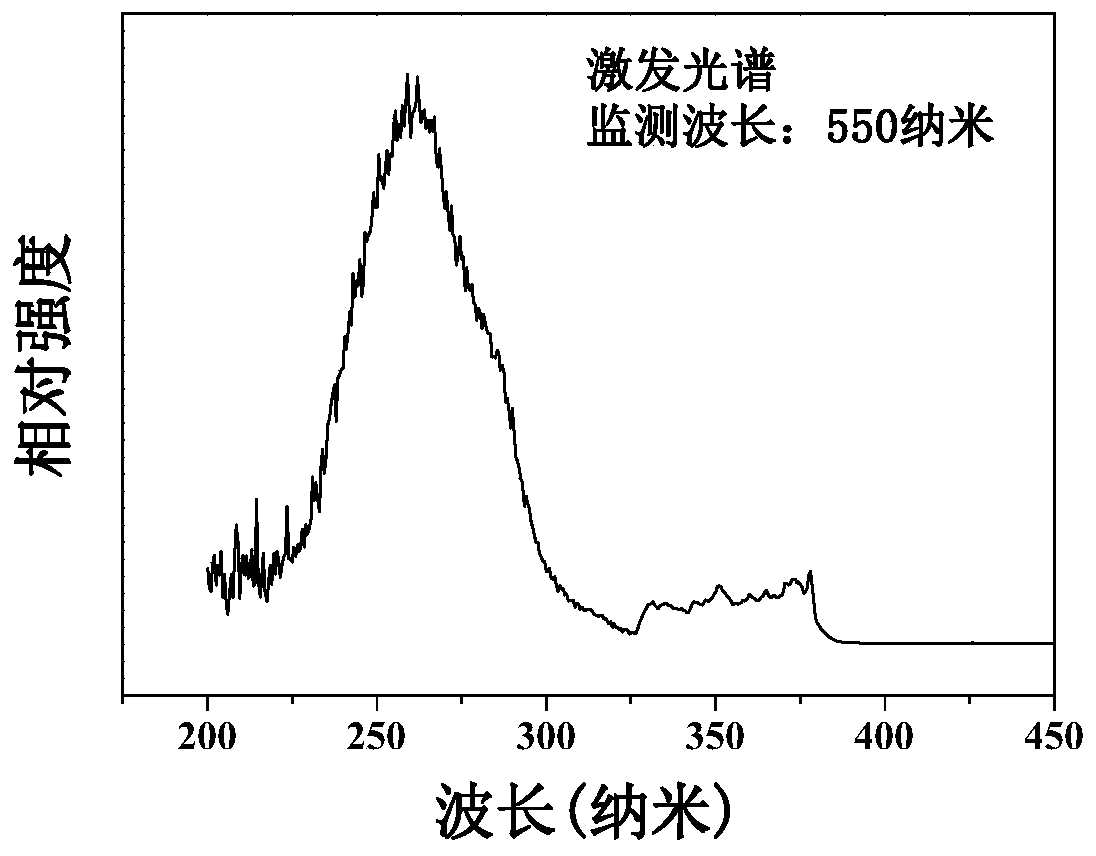
[0038] The sample prepared in this example is similar to Example 1 in its main structural appearance, excitation spectrum, emission spectrum and luminescence deca...
Embodiment 3
[0040] Preparation of Ca 9.5 Tb 0.5 Si 3 o 15 f 2 , according to the chemical formula Ca 9.5 Tb 0.5 Si 3 o 15 f 2 The stoichiometric ratio of each element in , respectively weigh calcium oxalate CaC 2 o 4 : 9.608 g, silicic acid H 2 SiO 3 : 2.34 g, terbium nitrate Tb (NO 3 ) 3 ·6H 2 O: 0.0226 g, after grinding and mixing in an agate mortar, select the air atmosphere for pre-sintering, the sintering temperature is 900 ° C, the sintering time is 6 hours, then cool to room temperature, take out the sample; with 1.5616 g calcium fluoride CaF 2 Fully mix and grind evenly, press and form the mixed powder at a pressure of 12 MPa, and calcinate again in an air atmosphere at a calcining temperature of 1000°C for 9 hours, cool naturally, grind and mix evenly to obtain a fluorescent ceramic material.
[0041] The sample prepared in this example is similar to Example 1 in its main structural appearance, excitation spectrum, emission spectrum and luminescence decay curve. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com