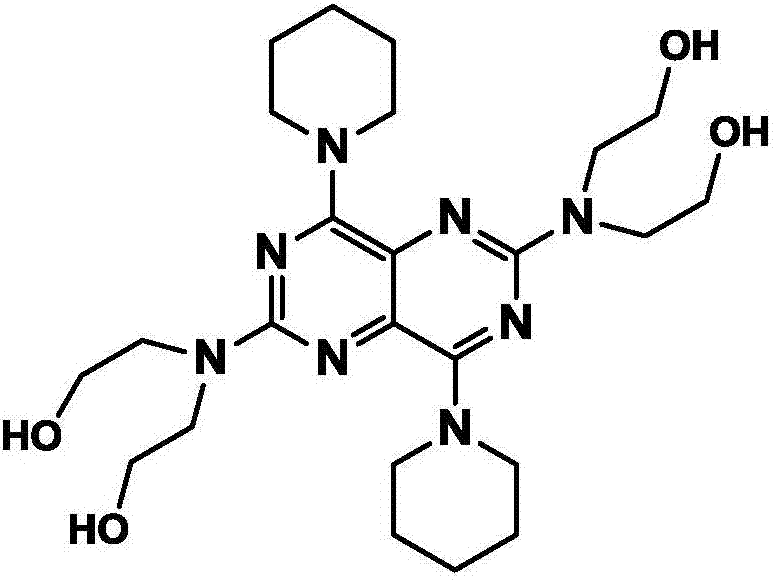
Novel technology with introduced catalyst to optimize synthesis of dipyridamole
A dipyridamole and new process technology, applied in the field of pharmaceutical intermediates, can solve the problems of serious environmental pollution, easy oxidative decomposition, difficult post-processing, etc., achieve simple post-processing, easy operation, and improve environmental pollution and operational danger. effect of the problem
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
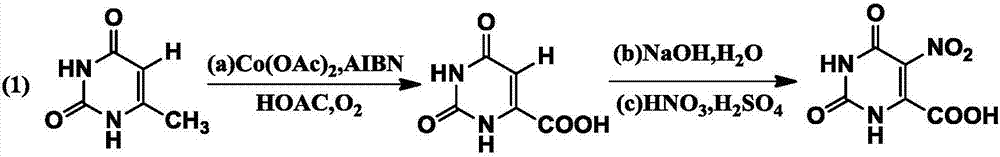
[0023] Example 1: Synthesis of 5-nitro-2,6-dioxo-1,3-dihydropyrimidine-4-carboxylic acid
[0024]
[0025] (a) In a 500ml three-necked flask, sequentially add 6-methylpyrimidine-2,4(1H,3H)-dione (10.0g, 79.4mmol), AIBN (azobisisobutyronitrile, 0.26g, 1.6mmol ), cobalt acetate (0.28g, 1.6mmol), glacial acetic acid 250ml, and oxygen (flow rate is 6-10m / s, diameter area is 9×10 -6 m 2 ), heated to 80°C for 15 hours, after the reaction, poured into saturated brine, filtered under reduced pressure, dried, recrystallized (good solvent chloroform, poor solvent methanol) to obtain 11.4g of white solid, the yield was 92.0 %. MS(EI):m / z:156.0169([M] + ).
[0026] (b) In a 500ml three-necked flask, add 2,6-dioxo-1,3,5-trihydropyrimidine-4-carboxylic acid (11.4g, 73.1mmol), 125ml of NaOH aqueous solution (500g sodium hydroxide +1500g purified water), stirred for 3h;
[0027] (c) Add nitric acid into a 500ml three-necked flask, cool it at minus 25°C with liquid nitrogen, then slow...
Embodiment 2
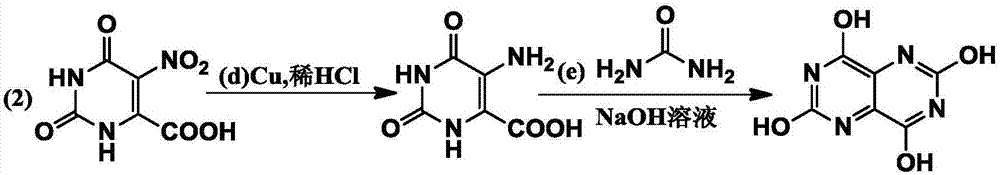
[0028] Example 2: Synthesis of pyrimido[5,4-d]pyrimidine-2,4,6,8-tetraol
[0029]
[0030] d) Substrate 5-nitro-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid (13.0g, 64.7mmol), activated copper powder (8.28g , 129.4mmol), was added to a 500ml dry two-necked flask, and 200ml of dilute hydrochloric acid (volume ratio of concentrated hydrochloric acid: water = 1:10) was added as a reaction solvent. The reaction solution was extracted with methyl chloride, and after the solvent was evaporated by rotary evaporation, it was directly put into the next step of reaction;
[0031] e) Put 5-amino-2,6-dioxo-1,2,3,6-tetrahydropyrimidine-4-carboxylic acid and urea (7.7g, 128.0mmol) into a two-necked flask, and add 125ml of NaOH solution dropwise (500g sodium hydroxide + 1500g purified water), after the dropwise addition, the temperature was rapidly raised to 100°C, and kept for one hour; continued to stir for 3h, cooled to room temperature (25°C), filtered to obtain a solid, ...
Embodiment 3
[0033]
[0034](f) Add pyrimido[5,4-d]pyrimidine-2,4,6,8-tetraol (10.9g, 55.2mmol), SOCl2 (8.1ml, 110.0mmol, ρ=1.638g) into a 500 three-necked flask / ml), dioxane 200ml was used as the reaction solvent to react for 1 hour, and DMF (4.2ml, 55.2mmolρ=0.95g / ml) was added to react in dark for 1.5 hours. After the reaction, the solvent was removed under reduced pressure, the solid was extracted with hot toluene solution, and the crude product was subjected to column chromatography with PE:DCM=2:1 to obtain 13.1 g of white solid with a yield of 88.59%. MS(EI):m / z:267.8872([M] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com