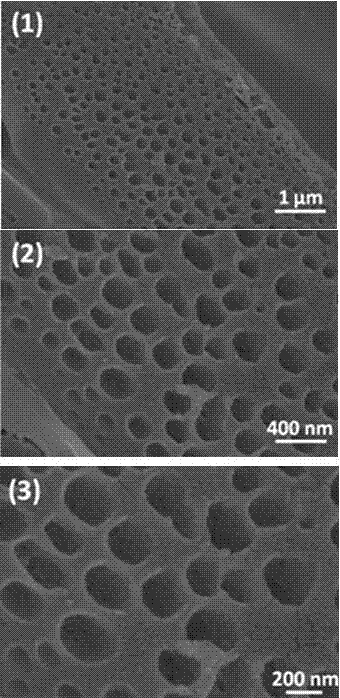
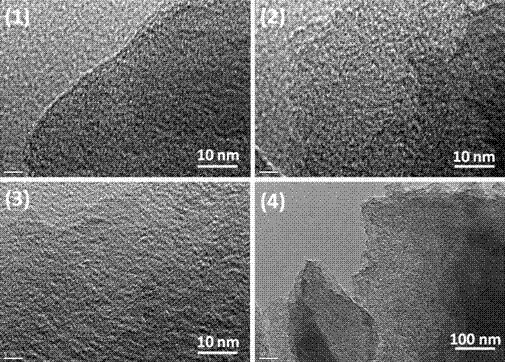
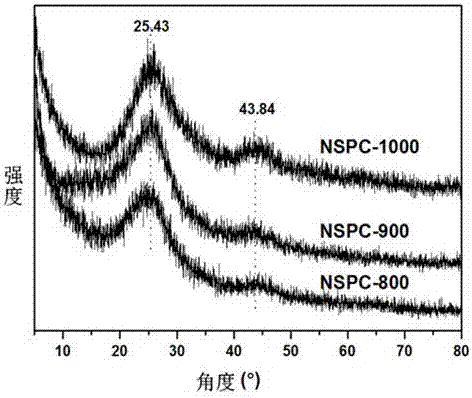
Preparation of N, S codoped graphitized carbon material and application as electrochemical catalyst
A technology of graphitized carbon and co-doping, which is applied in physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc. The effect of catalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Preparation of CMVImBr monomer: Add 1-vinylimidazole (9.411 g) and bromoacetonitrile (11.995 g, molar ratio 1:1) into a 50 ml round-bottomed flask and stir at room temperature for 4-5 hours to form Yellow solid; dissolve the solid in methanol, settle the solution in anhydrous ether, and finally filter to obtain a white solid, which is oven-dried at 30°C, which is CMVImBr monomer;
[0034] (2) Preparation of polyionic liquid PCMVImBr: Add CMVImBr monomer (10 g) and AIBN (0.2 g) initiator together into a 250 ml round bottom flask, then add 100 ml dimethyl sulfoxide as solvent, dissolve Finally, the reaction vessel was placed in an ice bath, and the whole reaction system was evacuated and nitrogen gas was applied, and this was repeated 3 times. After the solution in the round bottom flask reached room temperature, it was placed in an oil bath at 75° C. for 72 hours. After the polymerization is completed, the above solution is settled in tetrahydrofuran, and after cent...
Embodiment 2
[0040] (1) Preparation of CMVImBr monomer: same as Example 1;
[0041] (2) Preparation of polyionic liquid PCMVImBr: same as Example 1;
[0042] (3) Polymer 1-cyanomethyl-3-vinylimidazole bis(trifluoromethanesulfonyl)imide (PCMVImTf 2 N) preparation: same as Example 1;
[0043] (4) BSA@PCMVImTf 2 Preparation of N composite membrane: (4) BSA@PCMVImTf 2 Preparation of N composite membrane: PCMVImTf 2 N (0.1624 g) and BSA (0.1624 g, mass ratio 1:1) were dissolved in DMF (7 mL) to obtain a transparent homogeneous solution. Apply the solution on a glass slide and dry it in an oven at 75°C for 3 h, then soak the glass slide in 0.5 wt% ammonia ethanol solution for 2 hours to obtain BSA@PCMVImTf 2 N porous precursor film material;
[0044] (5) BSA@PCMVImTf 2 Preparation of N porous carbon: put the porous precursor film prepared above into a tube furnace and raise the temperature to 1000°C at a rate of 10°C / min, and keep it for 1 hour, and then naturally cool down to room temper...
Embodiment 3
[0046] (1) Preparation of CMVImBr monomer: same as Example 1;
[0047] (2) Preparation of polyionic liquid PCMVImBr: same as Example 1;
[0048] (3) Polymer 1-cyanomethyl-3-vinylimidazole bis(trifluoromethanesulfonyl)imide (PCMVImTf 2 N) preparation: same as Example 1;
[0049] (4) BSA@PCMVImTf 2 Preparation of N composite membrane: PCMVImTf 2 N (1.7864 g) and BSA (0.1624 g, mass ratio 11:1) were dissolved in DMF (7 mL) to obtain a transparent homogeneous solution. Apply the solution on a glass slide and dry it in an oven at 75°C for 3 h, then soak the glass slide in 0.5 wt% ammonia ethanol solution for 2 hours to obtain BSA@PCMVImTf 2 N porous precursor film material;
[0050] (5) BSA@PCMVImTf 2 Preparation of N porous carbon: put the porous precursor film prepared above into a tube furnace and raise the temperature to 1000°C at a rate of 10°C / min, and keep it for 1 hour, and then naturally cool down to room temperature to obtain BSA@PCMVImTf 2 N porous carbon material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com