Synthetic method of visible light catalytic 2-imine thiazoline derivatives
A technology of iminothiazoline and synthesis method is applied in the field of synthesis of 2-iminothiazoline derivatives, can solve the problems of harsh reaction conditions, low selectivity and high reaction temperature, and achieves mild reaction conditions and high yield Good, easy-to-use results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
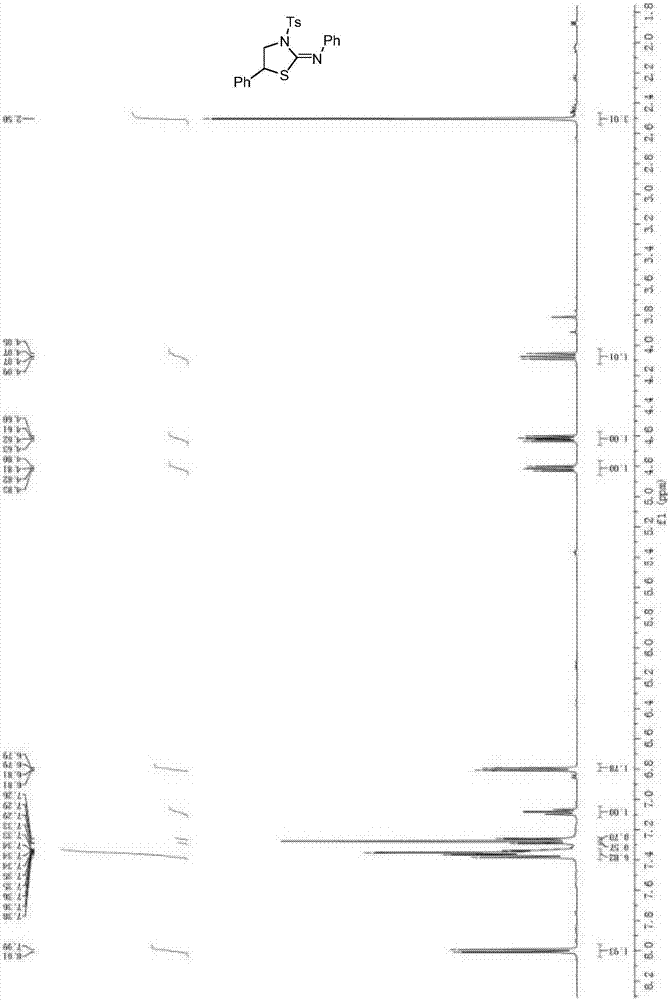
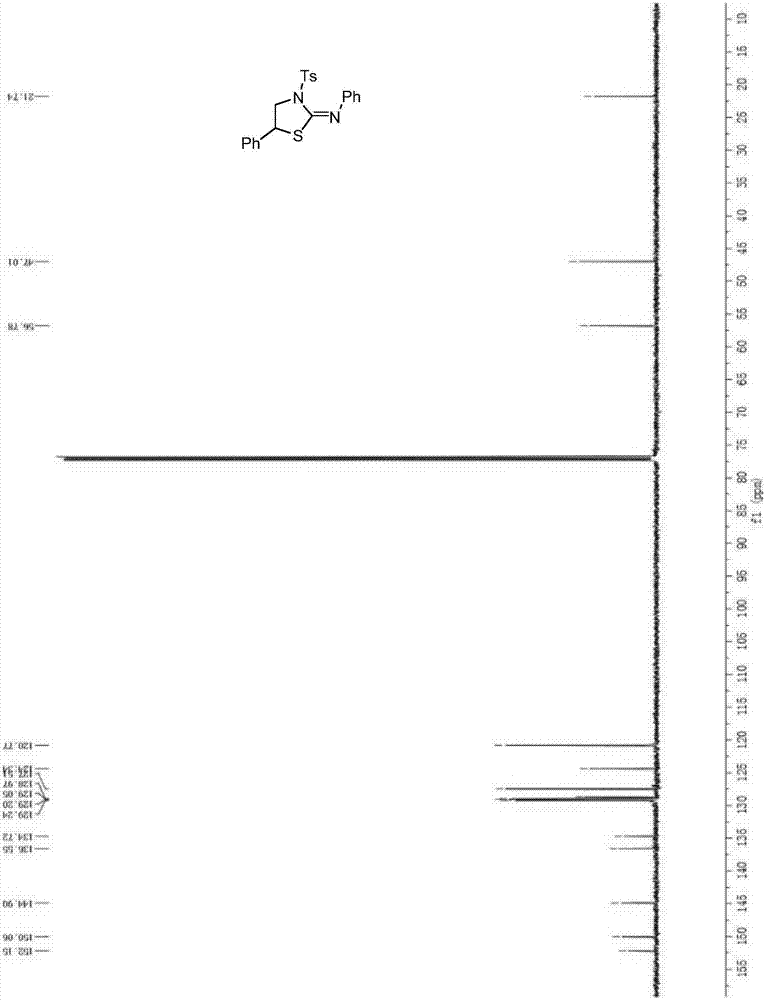
[0038] In a 25mL Schlenk tube, sequentially add 0.3mmol R 1 for hydrogen, R 2 Saturated aziridine for phenyl, 0.006mmol Ru(bpy) 3 (BF 4 ) 2 , 0.9mmol PhN 2 BF 4 , vacuum nitrogen three times, under the protection of nitrogen, add 4mL of anhydrous 1,2-dichloroethane, 0.3mmol phenyl isothiocyanate, and stir at room temperature for 10h under the irradiation of a 30W household white light energy-saving lamp. After the reaction is completed, it is separated and purified by column chromatography (column chromatography developer ethyl acetate / petroleum ether=1:10-20) to obtain N,5-diphenyl-3-p-methylbenzenesulfonylthiazole-2-ylidene Amine 112.6 mg, yield 91.9%.
[0039] Product characterization data are as follows:
[0040] 1 H NMR (500MHz, CDCl 3 )δ8.00(d, J=8.3Hz, 2H), 7.40-7.33(m, 7H), 7.29(d, J=1.9Hz, 1H), 7.26(s, 1H), 7.08(t, J=7.4 Hz,1H),6.80(dd,J=8.3,0.9Hz,2H),4.82(dd,J=8.5,6.4Hz,1H),4.62(dd,J=10.4,6.4Hz,1H),4.07(dd ,J=10.4,8.6Hz,1H),2.50(s,3H). 13 C NMR (126MHz, C...
Embodiment 2
[0043] In a 25mL Schlenk tube, sequentially add 0.3mmol R 1 for hydrogen, R 2 Saturated aziridine for phenyl, 0.006mmol Ru(bpy) 3 (BF 4 ) 2 , 0.9mmol PhN 2 BF 4 , vacuum nitrogen three times, under the protection of nitrogen, add 4mL of anhydrous ethylene glycol dimethyl ether (DME), 0.3mmol phenyl isothiocyanate, and stir at room temperature for 12h under the irradiation of a 30W household white light energy-saving lamp. After the reaction is completed, it is separated and purified by column chromatography (column chromatography developer ethyl acetate / petroleum ether=1:10-20) to obtain N,5-diphenyl-3-p-methylbenzenesulfonylthiazole-2-ylidene Amine 66.8 mg, yield 54.6%.
Embodiment 3
[0045] In a 25mL Schlenk tube, sequentially add 0.3mmol R 1 for hydrogen, R 2 Saturated aziridine for phenyl, 0.006mmol Ru(bpy) 3 (BF 4 ) 2 , 0.9mmol PhN 2 BF 4 , vacuum nitrogen three times, under the protection of nitrogen, add 4mL of anhydrous diethyl ether, 0.3mmol phenyl isothiocyanate, and stir at room temperature for 12h under the irradiation of a 30W household white light energy-saving lamp. After the reaction is completed, it is separated and purified by column chromatography (column chromatography developer ethyl acetate / petroleum ether=1:10-20) to obtain N,5-diphenyl-3-p-methylbenzenesulfonylthiazole-2-ylidene Amine 75.7 mg, yield 61.8%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com