Polysaccharide and bile salt lipidosome for oral administration and preparation method of lipidosome
A liposome and bile salt technology, which is applied in liposome delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve problems such as difficulty in oral absorption, difficulty in achieving ideal pharmacological activity in clinical application, and large molecular weight of polysaccharides , to achieve the effect of reducing damage, increasing in vitro stability, and increasing stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
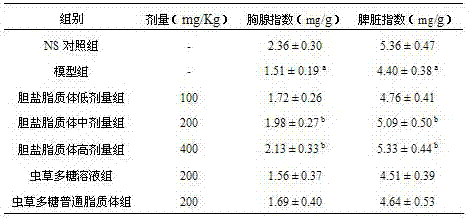
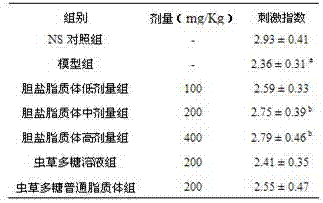
[0024] (1) Prescription composition: soybean lecithin 800 mg, cholesterol 100 mg, sodium deoxycholate 100 mg, cordyceps polysaccharide 80 mg.
[0025] (2) Preparation: Take 800 mg of soybean lecithin, 100 mg of cholesterol, and 100 mg of sodium deoxycholate, add 100 mL of dichloromethane, and dissolve to form a solution. Take 80 mg of Cordyceps polysaccharide, dissolve it in 30 mL of pH7.4 phosphate buffer, inject it into the dichloromethane solution, and pulse ultrasound for 3 min in ice-water bath to form a stable W / O emulsion. The organic solvent was removed by rotary evaporation at 30°C, and the liquid became a viscous colloid. Add 20 mL of 3% mannitol pH 6.8 phosphate buffer solution, and continue rotary steaming for 30 min. The colloids are dispersed in the solution to obtain a liposome suspension. The liposome suspension was subjected to pulse ultrasonication for 5 min, then homogenized through a high-pressure homogenizer (pressure 200 bar, cycle 3 times), and filtered...
Embodiment 2
[0030] (1) Prescription composition: hydrogenated soybean lecithin 800 mg, cholesterol 100 mg, sodium deoxycholate 100 mg, seaweed polysaccharide 80 mg.
[0031] (2) Preparation: Take 800 mg of hydrogenated soybean lecithin, 100 mg of cholesterol, and 100 mg of sodium deoxycholate, add 100 mL of dichloromethane, and dissolve to form a solution. Dissolve 80 mg of seaweed polysaccharide in 25 mL of pH 7.4 phosphate buffer, inject it into the methylene chloride solution, pulse ultrasonic in an ice-water bath for 3 min to form a stable W / O emulsion. The organic solvent was removed by rotary evaporation at 30°C, and the liquid became a viscous colloid. Add 20 mL of 3% mannitol pH 6.8 phosphate buffer solution, continue rotary steaming for 30 min, and colloids are dispersed in the solution to obtain a liposome suspension. The liposome suspension was subjected to pulse ultrasonication for 5 min, then homogenized through a high-pressure homogenizer (pressure 200 bar, cycle 3 times), ...
Embodiment 3
[0034] (1) Prescription composition: soybean lecithin 800 mg, cholesterol 100 mg, sodium glycocholate 100 mg, pine pollen polysaccharide 100 mg.
[0035] (2) Preparation: Take 800 mg of soybean lecithin, 100 mg of cholesterol, and 80 mg of sodium glycocholate, add 100 mL of chloroform, and dissolve to form a solution. Take 100 mg pine pollen polysaccharide, dissolve it in 30 mL pH7.4 phosphate buffer, inject it into the chloroform solution, and pulse ultrasonic for 5 min in an ice-water bath to form a stable W / O emulsion. The organic solvent was removed by rotary evaporation at 30°C, and the liquid became a viscous colloid. Add 25 mL of 3.2% mannitol pH 6.8 phosphate buffer solution, continue rotary evaporation for 30 min, and the colloids are dispersed in the solution. Pulse ultrasound for 5 min, homogenize with a high-pressure homogenizer (pressure 200 bar, cycle 3 times), and filter through a 0.45 μm microporous membrane to obtain a liposome solution. The filtrate was div...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com