Polymer-supported dual-core acid ion liquid catalyst and preparation and application thereof
A technology of ionic liquids and polymers, applied in the preparation of organic compounds, catalysts of organic compounds/hydrides/coordination complexes, catalysts for physical/chemical processes, etc. Easy to lose components and other problems, to achieve the effect of broad industrial application prospects, fast mass transfer rate, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation method of polymer-loaded double nucleic acid ionic liquid:
[0027] (1) Preparation of polychloromethylstyrene microspheres
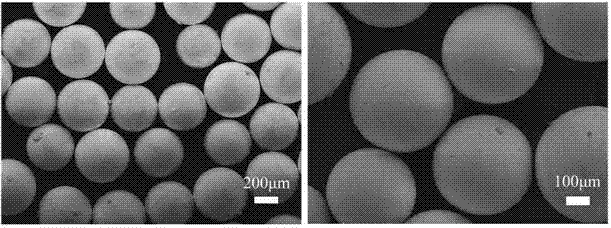
[0028] Add 6g of vinylbenzyl chloride, 2g of styrene, 3.2g of divinylbenzene, 0.1g of polyvinyl alcohol, 0.7g of azobisisobutyronitrile and 4.5g of n- Heptane, N 2 Under protection, reflux reaction at 70°C for 8 hours, the obtained product is suction filtered, washed, dried, and then sieved to obtain 20-200 mesh polymer microspheres;
[0029] (2) Polychloromethylstyrene microspheres loaded with ionic liquids
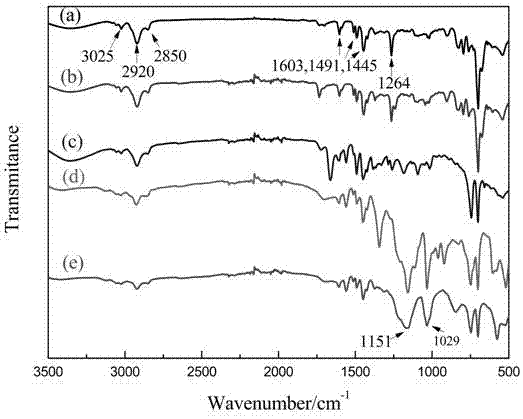
[0030] ① Using acetonitrile as a solvent, mix the above polymer carrier with bis(2-chloroethyl)amine at a mass ratio of 1.0:1.0, heat to 50°C, and react with magnetic stirring for 12 hours. Wash with acetonitrile, methanol, and ethanol, and dry in vacuum;
[0031] ②Using DMF as solvent, mix the product obtained in step ① with benzimidazole at a mass ratio of 1.0:1.0, heat to 75°C, and react with magnetic stirring for 24 ...
Embodiment 2
[0035] The preparation method of polymer-loaded double nucleic acid ionic liquid:
[0036] (1) Preparation of polychloromethylstyrene microspheres
[0037] Add 6g of vinylbenzyl chloride, 2g of styrene, 3.2g of divinylbenzene, 0.1g of polyvinyl alcohol, 0.7g of azobisisobutyronitrile and 6.7g of n- Heptane, N 2 Under protection, reflux reaction at 60°C for 8 hours, the obtained product can be obtained after suction filtration, washing, drying, and sieving to obtain 20-200 mesh polymer microspheres;
[0038] (2) Polychloromethylstyrene microspheres loaded with ionic liquids
[0039] ① Using acetonitrile as a solvent, mix the above polymer carrier with bis(2-chloroethyl)amine at a mass ratio of 1.0:1.0, heat to 40°C, and react with magnetic stirring for 12 hours. Wash with acetonitrile, methanol, and ethanol, and dry in vacuum;
[0040] ② Using DMF as a solvent, mix the product obtained in step ① with benzimidazole at a mass ratio of 1.0:1.0, heat to 90°C, and react with magne...
Embodiment 3
[0044] The preparation method of polymer-loaded double nucleic acid ionic liquid:
[0045] (1) Preparation of polychloromethylstyrene microspheres
[0046] Add 6g of vinylbenzyl chloride, 2g of styrene, 3.2g of divinylbenzene, 0.1g of polyvinyl alcohol, 0.7g of azobisisobutyronitrile and 6.7g of n- Heptane, under the protection of N2, reflux reaction at 80°C for 8 hours, the obtained product is suction filtered, washed, dried, and then sieved to obtain polymer microspheres of 20-200 mesh;
[0047] (2) Polychloromethylstyrene microspheres loaded with ionic liquids
[0048] ①Using acetonitrile as solvent, mix the above polymer carrier with bis(2-chloroethyl)amine at a mass ratio of 2.0:1.0, heat to 60°C, and react with magnetic stirring for 12 hours. Wash with acetonitrile, methanol, ethanol, etc., and dry in vacuum;
[0049] ② Using DMF as a solvent, mix the product obtained in step ① with benzimidazole at a mass ratio of 1.0:1.0, heat to 90°C, and react with magnetic stirri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com