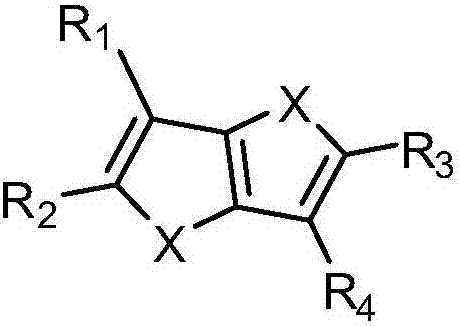
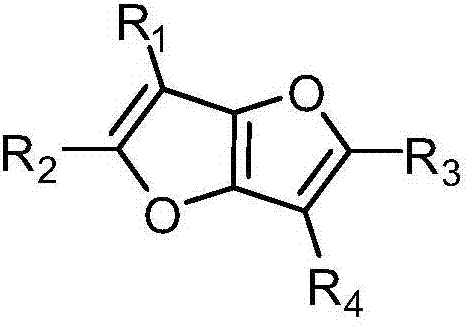
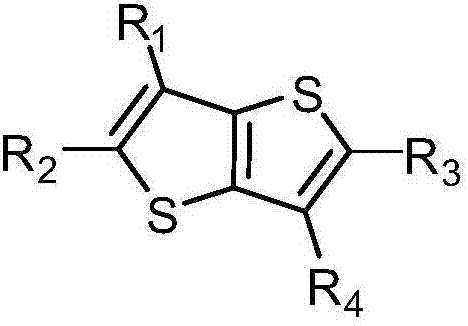
Thiophene or furan derivative and organic light emitting device using the derivative
A technology of organic light-emitting devices and furan derivatives, applied in the field of organic optoelectronic materials, can solve the problems of slower electron mobility than hole mobility, achieve high electron transport performance, solve carrier imbalance, and high luminous efficiency Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] [Example 1] Synthesis of compound 3-1
[0056] *Synthesis of Intermediate C-1-1
[0057]
[0058] Put into thieno[3,2-b]thiophene (4.21g, 30mmol) in the reactor, under nitrogen protection, add acetic acid 30ml, chloroform 50ml in the reactor, the Br of 20mmol 2 Slowly drop into the reactor to react, then stir for 1h, then gradually add 15mmol of Br 2 , stirred for 1 h, refluxed overnight, and cooled to room temperature to obtain a solid precipitate, which was washed with deionized water and methanol successively, dried, and the obtained crude product was passed through a silica gel column to obtain compound C-1-1 (12.72 g, 93%).
[0059] *Synthesis of Compound 3-1
[0060]
[0061] Add C-1-1 (1.91g, 4.2mmol), (4-cyanophenyl) boric acid (2.5g, 18.4mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate ( 5.3g, 38.3mmol), toluene 60mL, ethanol 20mL and distilled water 20mL, then stirred at 120°C for 3h. After the reaction was completed,...
Embodiment 2
[0062] [Example 2] Synthesis of compound 3-21
[0063] *Synthesis of Intermediate C-21-1
[0064]
[0065] Add 30ml of DMF and thieno[3,2-b]thiophene (2.31g, 16.5mmol) into the reactor, then cool down to 0°C, add NBS (8.81g, 49.5mmol), and react in the dark for 12h. After the reaction, use NaHSO 3 After washing, the solvent in the upper layer was removed, and the organic phase of the lower layer was concentrated and purified by silica gel column to obtain compound C-21-1 (4.67 g, 95%).
[0066] *Synthesis of compound 3-21
[0067]
[0068] Add C-21-1 (2.5g, 8.4mmol), anthraceneboronic acid (3.79g, 17.07mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate (5.3g, 38.3mmol) in the reaction vessel, Toluene 60mL, ethanol 20mL and distilled water 20mL were stirred at 120°C for 3h. After the reaction was completed, the reaction was stopped with distilled water and extracted with ethyl acetate. MgSO for organic layer 4 dry. The solvent was dist...
Embodiment 3
[0069] [Example 3] Synthesis of Compound 3-28
[0070] *Synthesis of Intermediate C-28-1
[0071]
[0072] Add C-1-1 (9.12g, 20mmol), acetic acid 500ml, toluene 200ml, Zn powder (2.62g, 40mmol) into the reactor, and slowly drop hydrochloric acid solution in the reaction process to stimulate the activity of Zn powder. After the reaction is completed, reflux After one night, then cooled to room temperature, the solution was concentrated and filtered to obtain compound C-28-1 (5.36 g, 90%). *Synthesis of intermediate C-28-2
[0073]
[0074] Add C-28-1 (2.5g, 8.4mmol), phenanthrene boric acid (3.79g, 17.07mmol), tetrakistriphenylphosphine palladium (0.7g, 1.08mmol), potassium carbonate (5.3g, 38.3mmol) in the reaction vessel, Toluene 60mL, ethanol 20mL and distilled water 20mL were stirred at 120°C for 3h. After the reaction was completed, the reaction was stopped with distilled water and extracted with ethyl acetate. MgSO for organic layer 4 dry. After the solvent wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
| Film thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap