Four-gear ring metal platinum (II) complex phosphorescent luminescent materials
A phosphorescent light-emitting material, metal platinum technology, applied in the direction of light-emitting materials, platinum group organic compounds, platinum group organic compounds, etc., can solve the problem of high luminescence color purity, achieve the effect of single structure, easy modification and adjustment, and energy reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
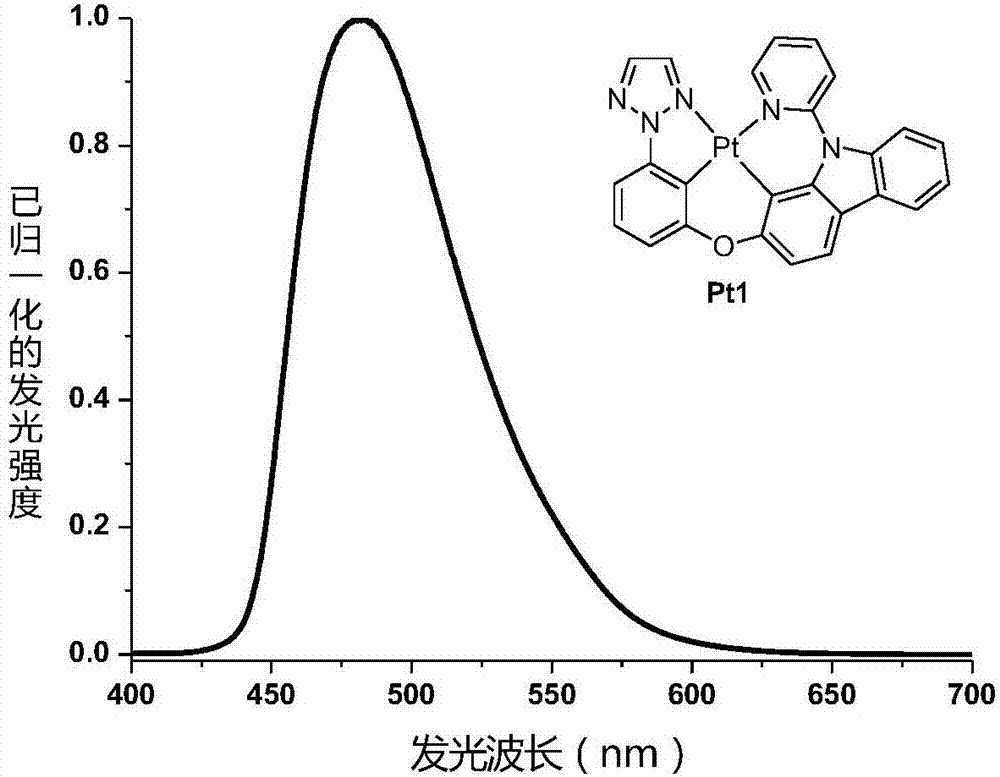
[0037] Example 1: The synthetic route of the phosphorescent material Pt1 of tetracyclic metal platinum complex is as follows:
[0038]
[0039] Synthesis of 2-(3-methoxyphenyl)-2H-1,2,3-triazole 1A and 1-(3-methoxyphenyl)-2H-1,2,3-triazole 1A' : Add 2H-1,2,3-triazole (2.04g, 30.0mmol, 1.0eq) and CuI (571.0mg, 3.0mmol, 0.10eq) to a 100mL three-neck flask with a magnetic rotor and a condenser, and prepare Body L-proline (690.0mg, 6mmol, 0.20eq) and K 2 CO 3 (8.29g, 60.0mmol, 2.0eq). The nitrogen was purged three times, then 3-methoxybromobenzene (7.53 mL, 60.0 mmol, 2.0 eq) and solvent dimethyl sulfoxide (60 mL) were added. The reaction mixture was placed in an oil bath at 120° C. for 3 days. Cool to room temperature, add 100 mL of water to quench the reaction, add 100 mL of ethyl acetate to dilute, filter with diatomaceous earth, and wash thoroughly with ethyl acetate. The organic phase was dried over anhydrous sodium sulfate. Filtration, the solvent was distilled off ...
Embodiment 2
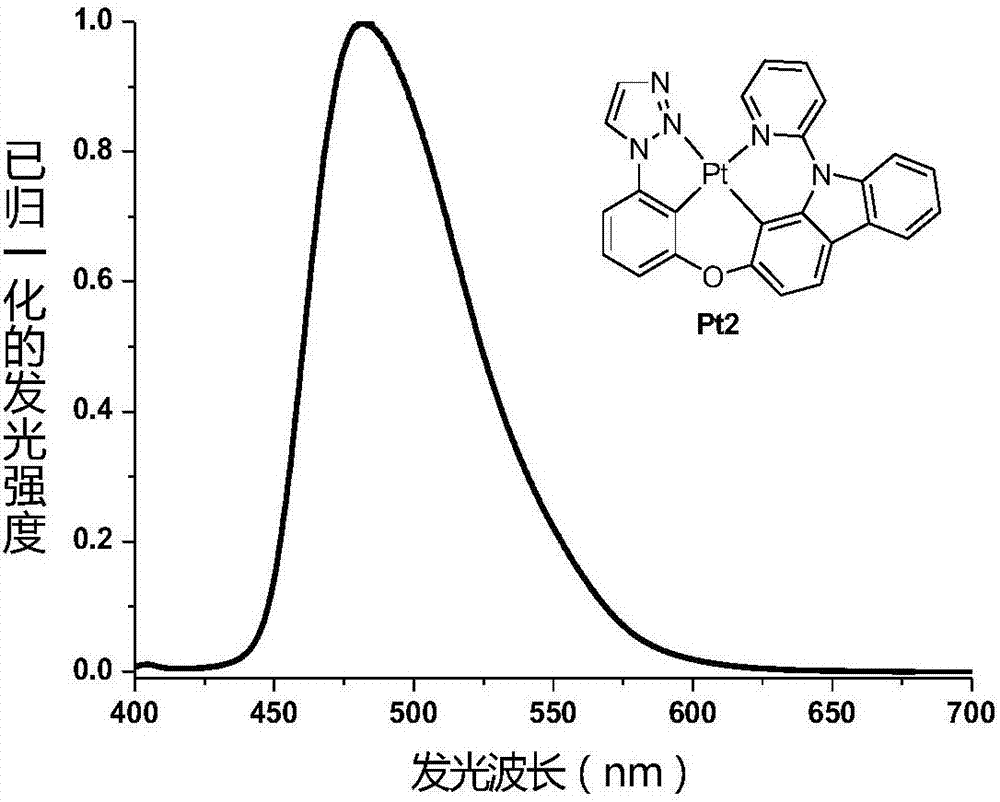
[0043] Embodiment 2: The synthesis route of tetradentate ring metal platinum complex phosphorescent material Pt2 is as follows:
[0044]
[0045] Synthesis of 1-(3-hydroxyphenyl)-1H-1,2,3-triazole 1B: 1-(3-methoxyphenyl)-1H-1,2,3- Triazole 1A' (1.36g, 7.70mmol) and concentrated hydrobromic acid (20.0mL, concentration 48%) and acetic acid (40.0mL) were refluxed at 120°C for 2 days in a one-necked flask equipped with a magnetic stirrer and a condenser . Then the mixture was allowed to cool to room temperature naturally, and the organic solvent and water were distilled off under reduced pressure, and the resulting mixture was neutralized with sodium bicarbonate solution until no bubbles occurred. Extracted with ethyl acetate, dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 1.24 g of the target product as a brown solid, with a yield of 99%. 1 HNMR (500MHz, DMSO-d 6 ): δ6.86-6.89(m,1H),7.30-7.31(m,2H),7.37-7.39...
Embodiment 3
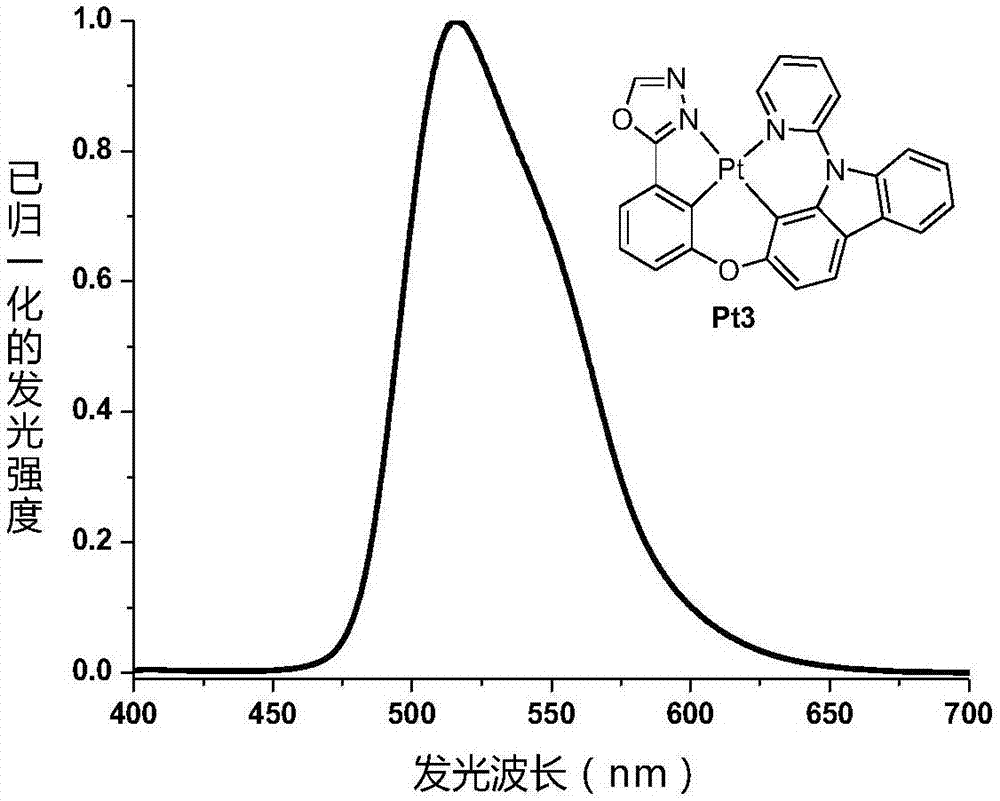
[0048] Example 3: The synthetic route of tetradentate ring metal platinum complex phosphorescent material Pt3 is as follows:
[0049]
[0050] Synthesis of 2-(3-hydroxyphenyl)-1,3,4-oxadiazole:
[0051] A mixture of methyl 3-hydroxybenzoate (6.85g, 45.0mmol), hydrazine hydrate (11.2mL, 80% aqueous solution) and ethanol (60mL) was heated to reflux for 48 hours, then cooled to room temperature, the precipitate was filtered out, washed with cold water, After drying, 4.34 g of the target product 3-hydroxybenzoic hydrazide was obtained with a yield of 63%. Then the obtained 3-hydroxybenzoic hydrazide (4.34 g, 28.5 mmol) was mixed with trimethoxymethane (19.7 mL), and heated to reflux for 24 hours. After cooling, excess trimethoxymethane was removed under reduced pressure, and the resulting crude product was separated and purified by silica gel column chromatography to obtain 4.24 g of the target product with a yield of 92%. 1 H NMR (500MHz, DMSO-d 6 ):δ7.01-7.03(m,1H),7.40-7....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com