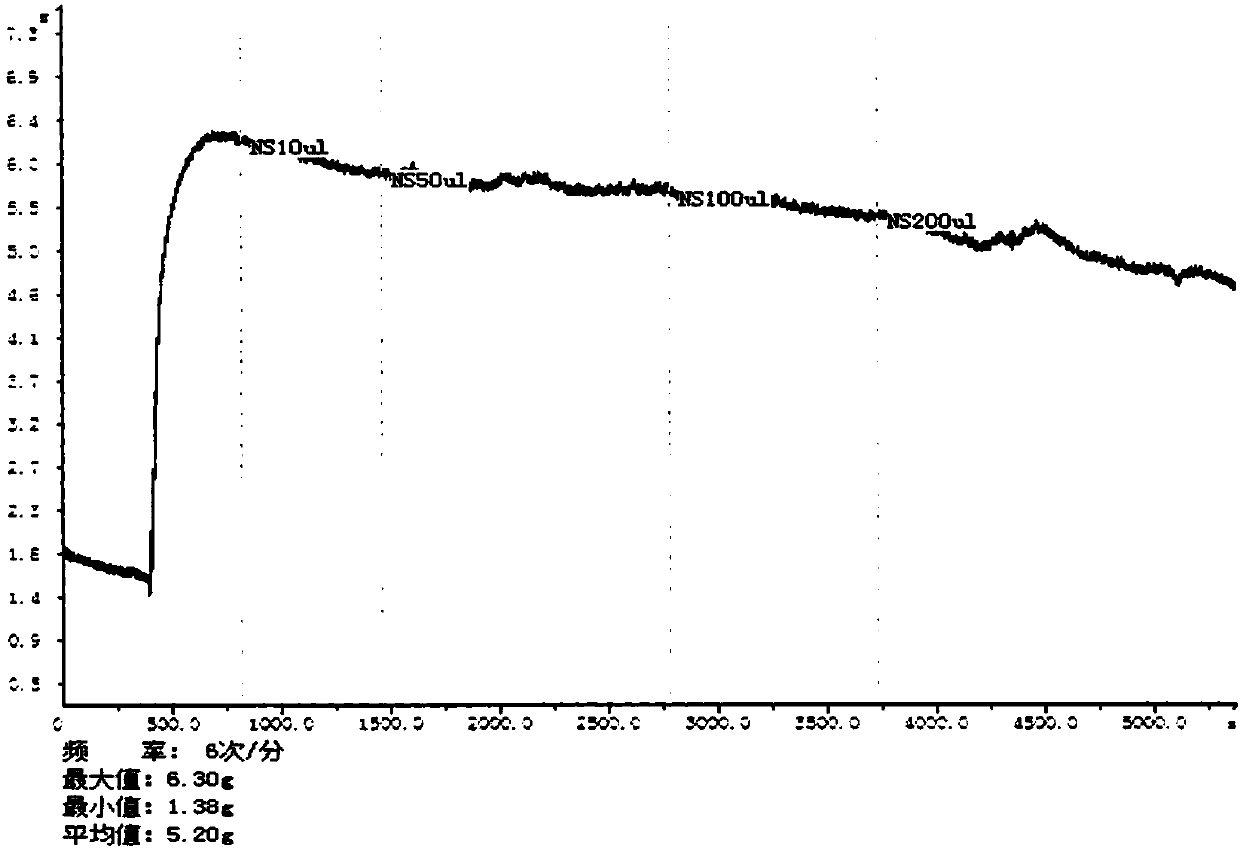
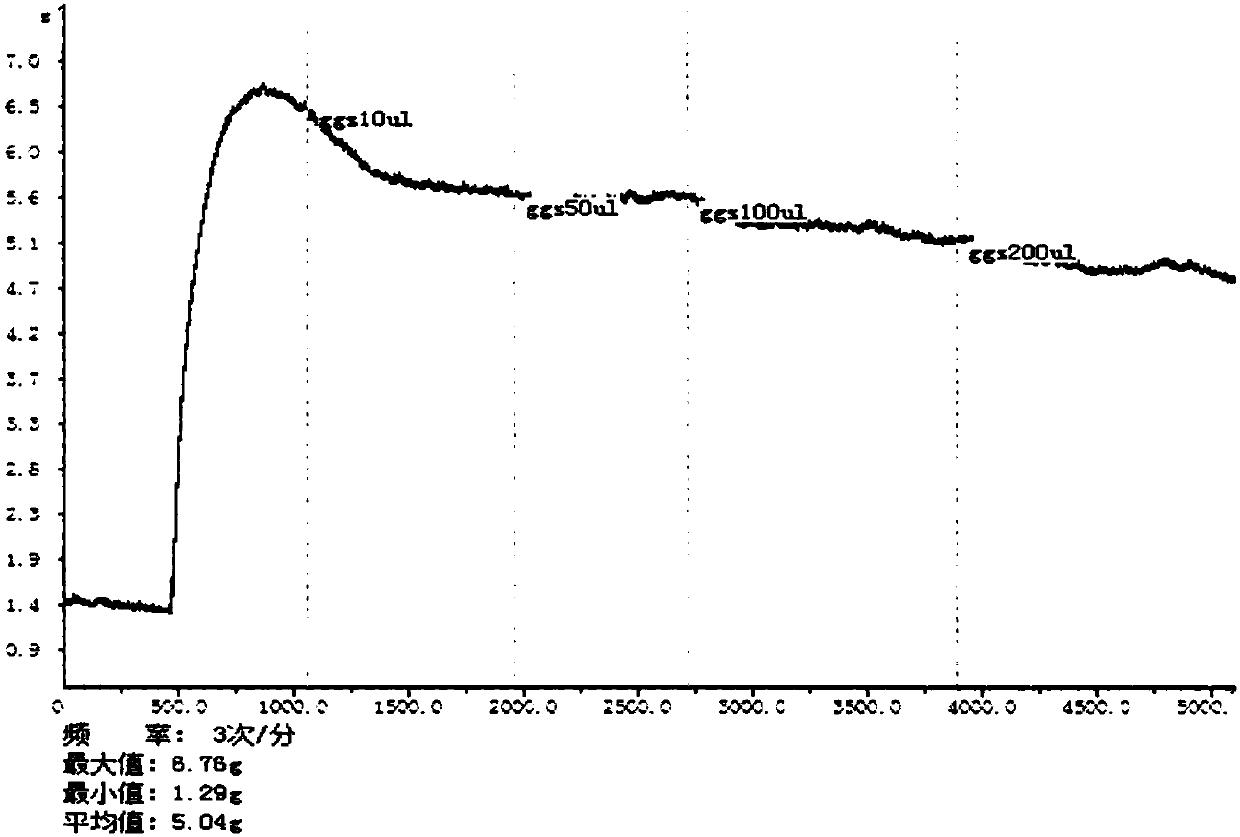
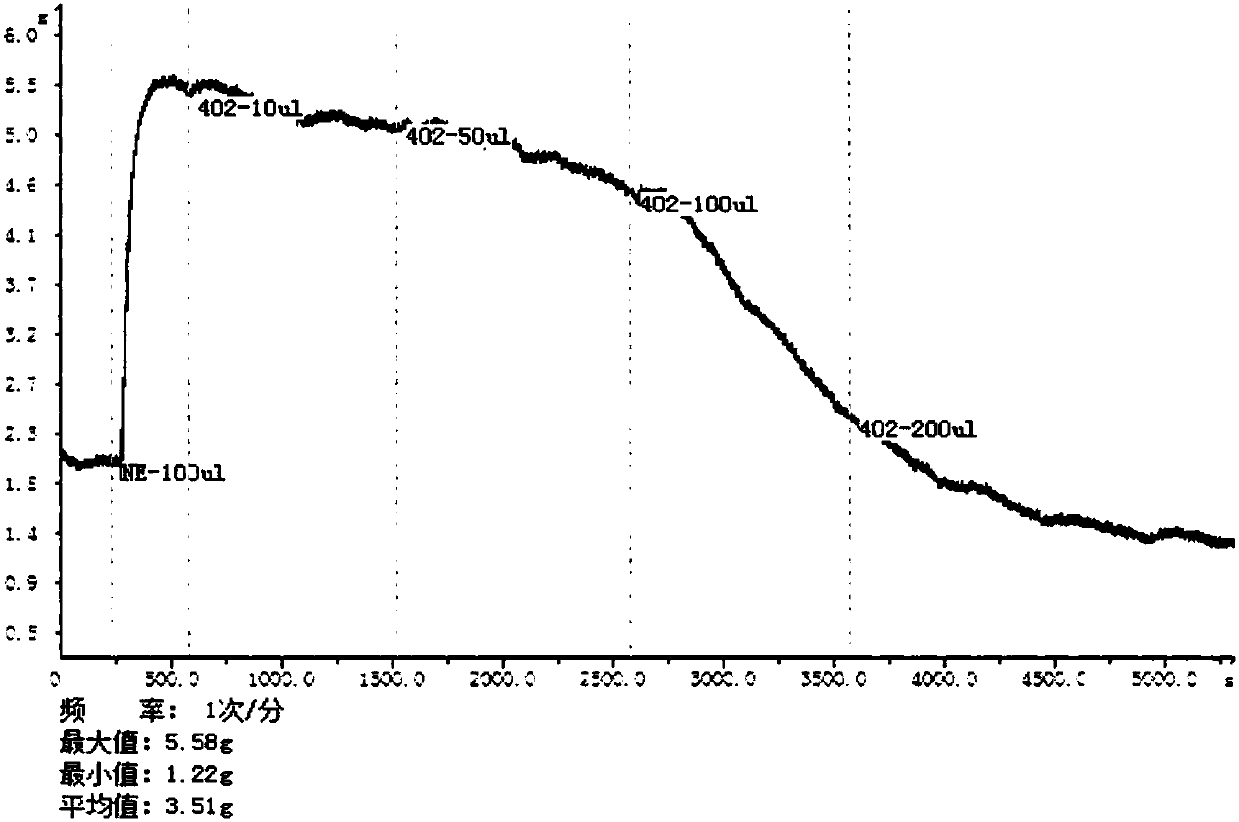
Puerarin derivative, preparation method thereof, and application thereof in preventing and treating cardiovascular and cerebrovascular diseases or diabetes mellitus or its complications
A technology of puerarin and drugs, applied in the field of cardiovascular and cerebrovascular and diabetes treatment, can solve problems such as poor water solubility, and achieve the effects of improving solubility, arterial contraction and relaxation, and improving physiological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment one: the structure of the compound of the present invention is as follows:
[0032]
[0033] The pharmaceutically acceptable salts of the above-mentioned compounds are the salts formed between the compound and inorganic bases, such as potassium hydroxide, sodium hydroxide, potassium carbonate, sodium carbonate, calcium chloride, calcium acetate or magnesium chloride, or with organic bases, such as amino Salts of butanetriol, aminoethanol, lysine or arginine, etc.
Embodiment 2
[0034] Embodiment two: the preparation of compound 1 and 2
[0035]
[0036] Weigh the compound puerarin (III) and place it in a dry round bottom flask, add ethyl acetate to dissolve it, then add 10% palladium carbon, stir and react at room temperature for 6 hours, TLC monitors the reaction process, after the reaction, filter the palladium carbon , the solvent was evaporated, and the obtained product was passed through a Flash reversed-phase column, and the mobile phase was methanol / water gradient elution, and finally the solvent was evaporated under reduced pressure to obtain compounds 1 and 2 as white solids.
[0037] Compound 1: MS m / z: 419 (M+1); 1 H NMR (400MHz, MeOD) δ7.75(d, J=8.8Hz, 1H), 7.08(d, J=8.6Hz, 2H), 6.75(d, J=8.5Hz, 2H), 6.54(d, J =8.8Hz,1H),4.93–4.88(m,2H),4.63–4.52(m,2H),4.04(s,1H),3.87(dd,J=11.9,1.9Hz,1H),3.73(dd, J=11.9,4.9Hz,1H),3.50–3.36(m,3H).
[0038] Compound 2: MS m / z: 419 (M+1); 1 H NMR (400MHz, MeOD) δ7.75(d, J=8.8Hz, 1H), 7.12(d, J=8.5Hz, 2H...
Embodiment 3
[0039] Embodiment three: the preparation of compound 3 and 4
[0040]
[0041] Weigh the compound dehydrated puerarin (V) and place it in a dry round bottom flask, add ethyl acetate to dissolve it, then add 10% palladium carbon, stir and react at room temperature for 6 hours, TLC monitors the reaction process, after the reaction, filter out the palladium carbon, the solvent was distilled off, and the obtained product was passed through a Flash reverse-phase column, and the mobile phase was methanol / water gradient elution, and finally the solvent was distilled off under reduced pressure to obtain compounds 3 and 4 as white solids.
[0042] Compound 3: MS m / z: 401 (M+1); 1 H NMR (600MHz, CD 3 OD) δ7.92(d, J=8.6Hz, 1H), 7.08(d, J=8.6Hz, 2H), 6.77(d, J=8.6Hz, 2H), 6.68(d, J=8.6Hz, 1H ),5.18(d,J=3.3Hz,1H),4.70–4.55(m,3H),4.00-3.97(m,2H),3.84(dd,J=12.1,2.5Hz,1H),3.64(dd, J=12.1,5.7Hz,1H),3.60(t,J=9.4Hz,1H),3.35(ddd,J=9.3,5.7,2.3Hz,1H).
[0043] Compound 4: MS m / z: 401 (M+1); ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com