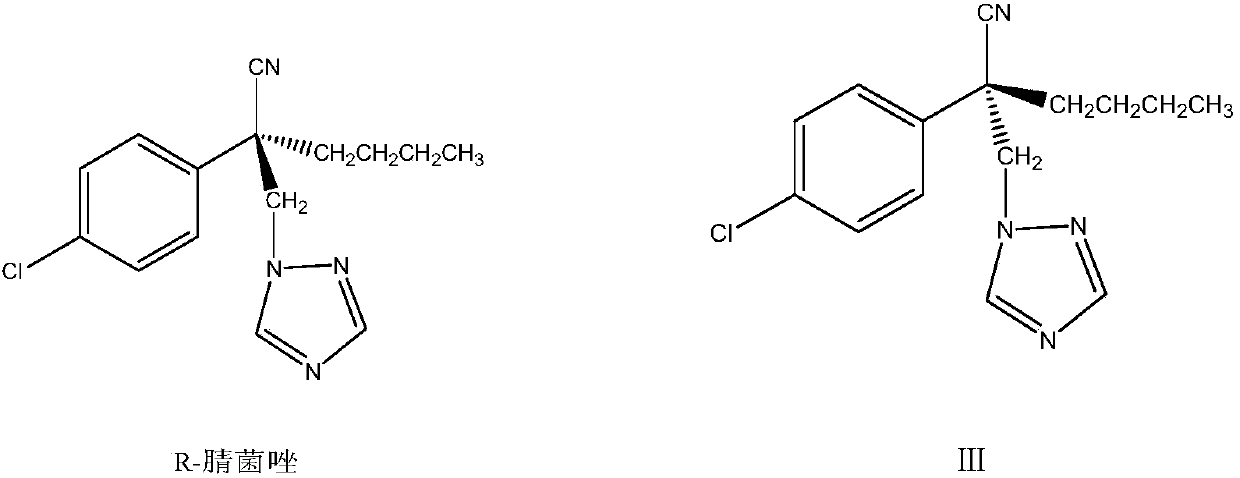
A kind of preparation method of highly active myclobutanil
A technology of myclobutanil and high activity is applied in the field of preparation of high-activity myclobutanil, which can solve the problems of complex splitting, achieve high yield and purity, reduce by-products, and avoid the effects of cyano hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The highly active solid base catalyst used in the following examples can be prepared according to the following methods:
[0038] Select according to the ratio of each raw material in the high activity solid catalyst, take 100g gamma-Al 2 o 3 As a carrier, add 2g of aluminum sol as a binder, and then add 15g of magnesium acetate (Mg(OAc) 2 ), after mixing and grinding for 1 hour, add 10 calcium hydroxide and 1g inorganic base, the inorganic base is alkali metal hydroxide, wherein the inorganic base can be sodium hydroxide or potassium hydroxide, then add 3g polyacrylamide as extrusion aid Agent, the molecular weight is between 11 million and 13 million, mixed and ground for 2 hours, added 20g of water, extruded into strips, dried, and finally placed in a muffle furnace and roasted at 500°C-600°C. Carry out calcining treatment 4 hours in the embodiment, obtain the composite solid base catalyst of high activity, standby.
Embodiment 2
[0040] The dimethyl sulfoxide solution of potassium triazole used in the following examples can be prepared according to the following method:
[0041] Add 80m of dimethyl sulfoxide and 25mg of toluene solvent in the reaction vessel, then add 125mg of triazole and 98mg of solid potassium hydroxide, heat, raise the temperature to reflux and carry out reflux dehydration reaction until the reaction is complete to obtain potassium triazole The dimethyl sulfoxide solution, so that the water content of the obtained potassium triazole dimethyl sulfoxide solution is less than or equal to 0.1%.
Embodiment 3
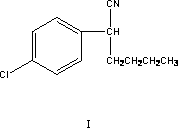
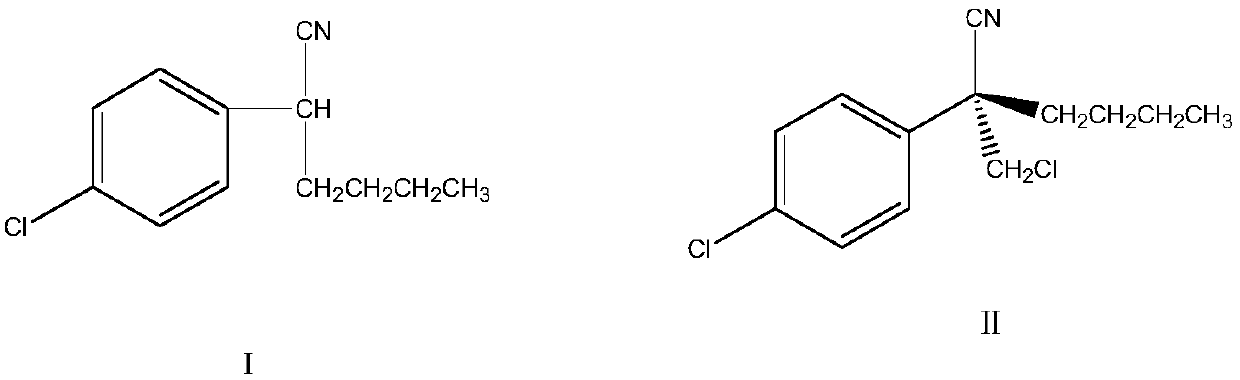
[0043] Add 300 mg of benzyl p-chloronitrile, 1500 mg of organic solvent toluene, 410 mg of bromo-n-butane, and 30 mg of highly active solid base catalyst into the three-necked flask of the reaction vessel, and raise the temperature. Then, the temperature was raised to 50°C ± 2°C for another three hours of reaction. After the reaction was completed, the temperature was slowly lowered to room temperature, filtered, and the solids were filtered off. The filtrate was collected and rectified under reduced pressure to remove the solvent to obtain intermediate 2-( 4-Chlorophenyl) hexonitrile, the yield is 91%, and the content reaches more than 99%.
[0044] Add the intermediate 2-(4-chlorophenyl) hexonitrile 300mg, chiral quaternary ammonium salt phase-transfer catalyst cinchonidine 3mg and methylene chloride 250mg obtained above in another three-necked flask of reaction vessel again, the added here Dichloromethane can be used both as a reaction raw material and as a reaction solvent...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com