Biological membrane stent material with gradient degradation effect and preparation method thereof
A scaffold material and biofilm technology, which is applied in medical science, textiles, papermaking, prostheses, etc., can solve the problems affecting the transportation of nutrients and metabolites, and the difficulty of extracting or synthesizing biologically active factors, so as to achieve superior performance and good biological performance. Effect of compatibility, fast degradation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
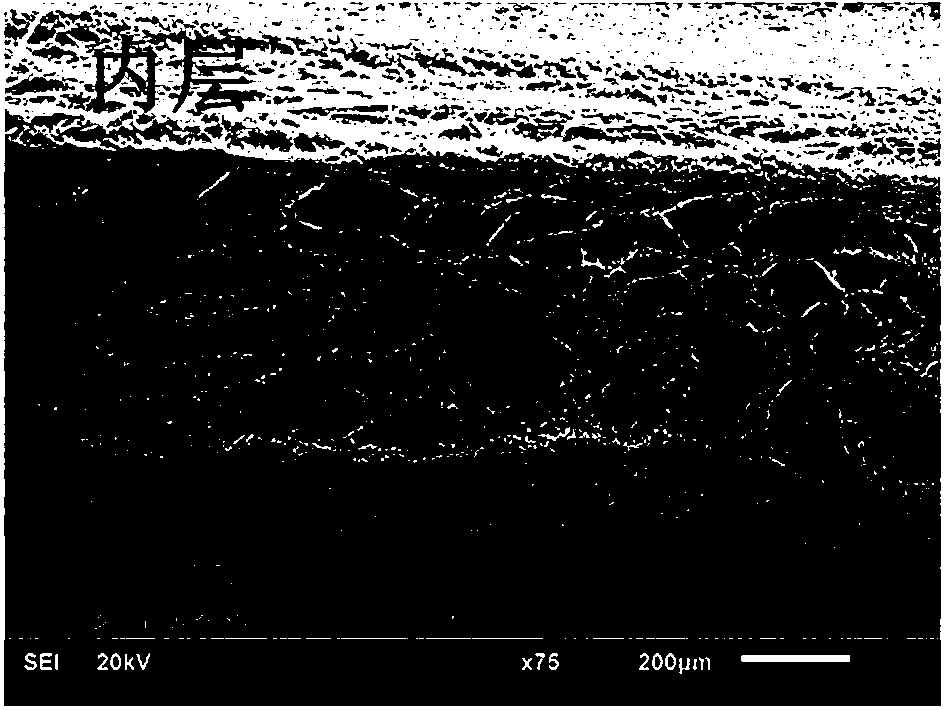
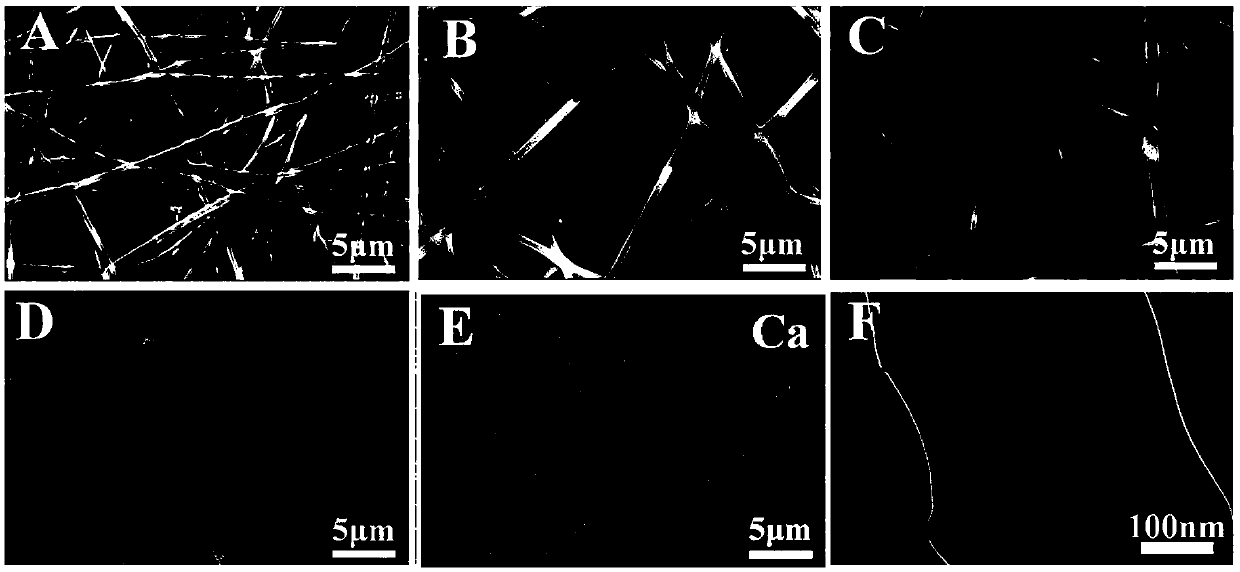
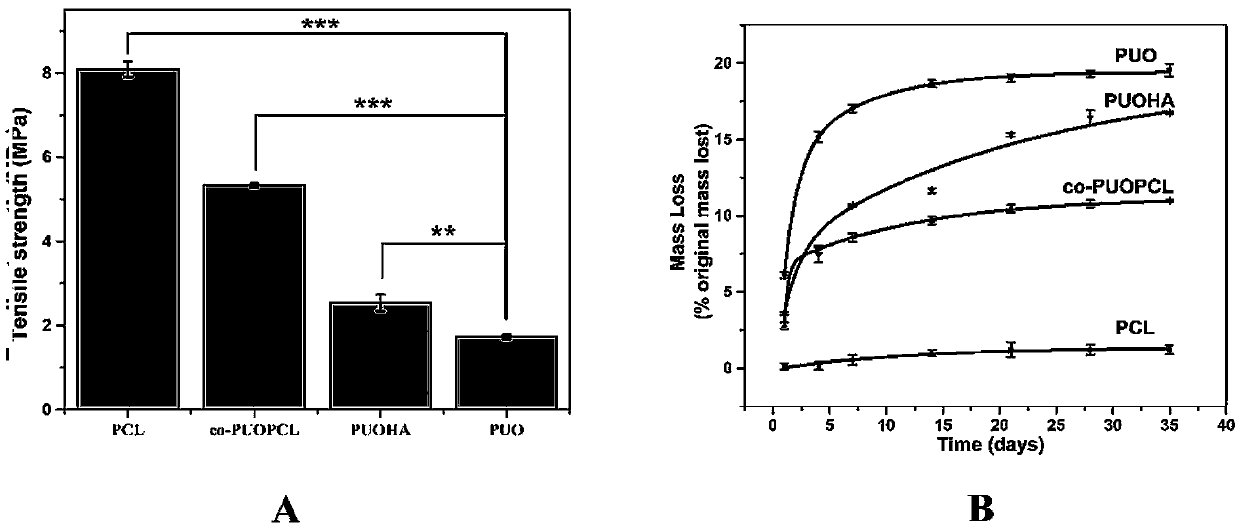
Image
Examples
Embodiment 1
[0041] (1) Preparation of polycaprolactone (PCL) electrospinning solution
[0042] Weigh 0.6 g of PCL particles, dissolve them in 5 mL of trifluoroethanol, add 1.2 mg of calcium chloride, and stir for 1 hour to obtain polycaprolactone spinning solution.
[0043] (2) Preparation of polyurethane (PU) electrospinning solution
[0044] Polyurethane synthesis: Prepolymerize 20g PCL (molecular weight 2000) and 11.1g isophorone diisocyanate (IPDI) (molecular weight 222.28) under mechanical stirring, nitrogen atmosphere, and 75℃ for 4 h, then add about 0.1 mL of stannous octoate Continue to stir for 1 h, then add 5 g of lysine ethyl ester hydrochloride and continue to react for 3 h, then add about 0.1 mL of stannous octoate to react for 2 h, then add 10 mL of deionized water, and place the reaction system at 90°C for 2 h. , The degradable medical polyurethane material target product can be obtained by cooling at room temperature.
[0045] Polyurethane composite (PUO) electrospinning solution...
Embodiment 2
[0051] (1) Preparation of polycaprolactone electrospinning solution
[0052] Weigh 0.7 g of PCL particles, dissolve them in 5 mL of trifluoroethanol, add 2.1 mg of calcium nitrate, and stir for 1 h to obtain polycaprolactone spinning solution.
[0053] (2) Preparation of polyurethane electrospinning solution and polyurethane / n-HA mixed electrospinning solution
[0054] Polyurethane synthesis: 20g PCL (molecular weight 2000) and 11.1g isophorone diisocyanate (IPDI) (molecular weight 222.28) were pre-polymerized for 6 h under mechanical stirring, nitrogen atmosphere, and 75°C; then about 0.15 mL stannous octoate was added Continue to stir for 1 h, then add 5 g of lysine ethyl ester hydrochloride and continue to react for 3 h, then add about 0.15 mL of stannous octoate to react for 3 h, then add 10 mL of deionized water and place the reaction system at 90°C for maturation After 10 hours, the target product of degradable polyurethane can be obtained by cooling at room temperature.
[005...
Embodiment 3
[0065] (1) Preparation of polycaprolactone electrospinning solution
[0066] Weigh 0.8 g of PCL particles, dissolve them in 5 mL of dichloromethane, add 3.2 mg of calcium bromide, and stir for 1 h to obtain polycaprolactone spinning solution.
[0067] (2) Preparation of composite polyurethane electrospinning solution
[0068] Polyurethane synthesis: 40 g of polycaprolactone diol (molecular weight 4000) and 9.05 g of lysine diisocyanate (LDI) (molecular weight 226.23) were pre-polymerized under mechanical stirring, nitrogen atmosphere, and 40°C for 10 h, and then added approximately 0.15 mL of stannous chloride was stirred for 2 hours, then 8g of PEG (molecular weight 400) was added for 4 hours, and then about 0.15 mL of stannous chloride was added to react for 3 hours, and then 8 mL of deionized water was added and the reaction system was placed at 70 The target product of degradable polyurethane can be obtained by curing at ℃ for 10 h and cooling at room temperature.
[0069] Compos...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com