Preparation method of nano alpha-Fe2O3
A nano and iron salt technology, applied in the direction of nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of irregular product shape, complicated operation, low reaction rate, etc., and achieve product shape Regular, simple process, good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
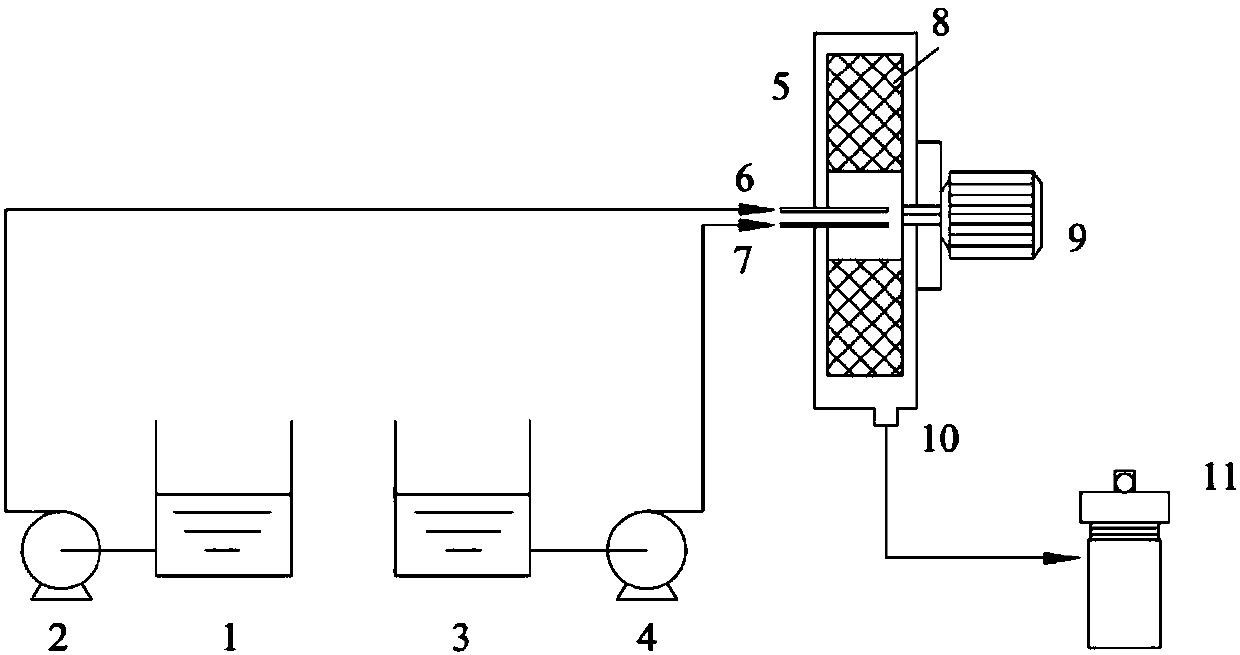
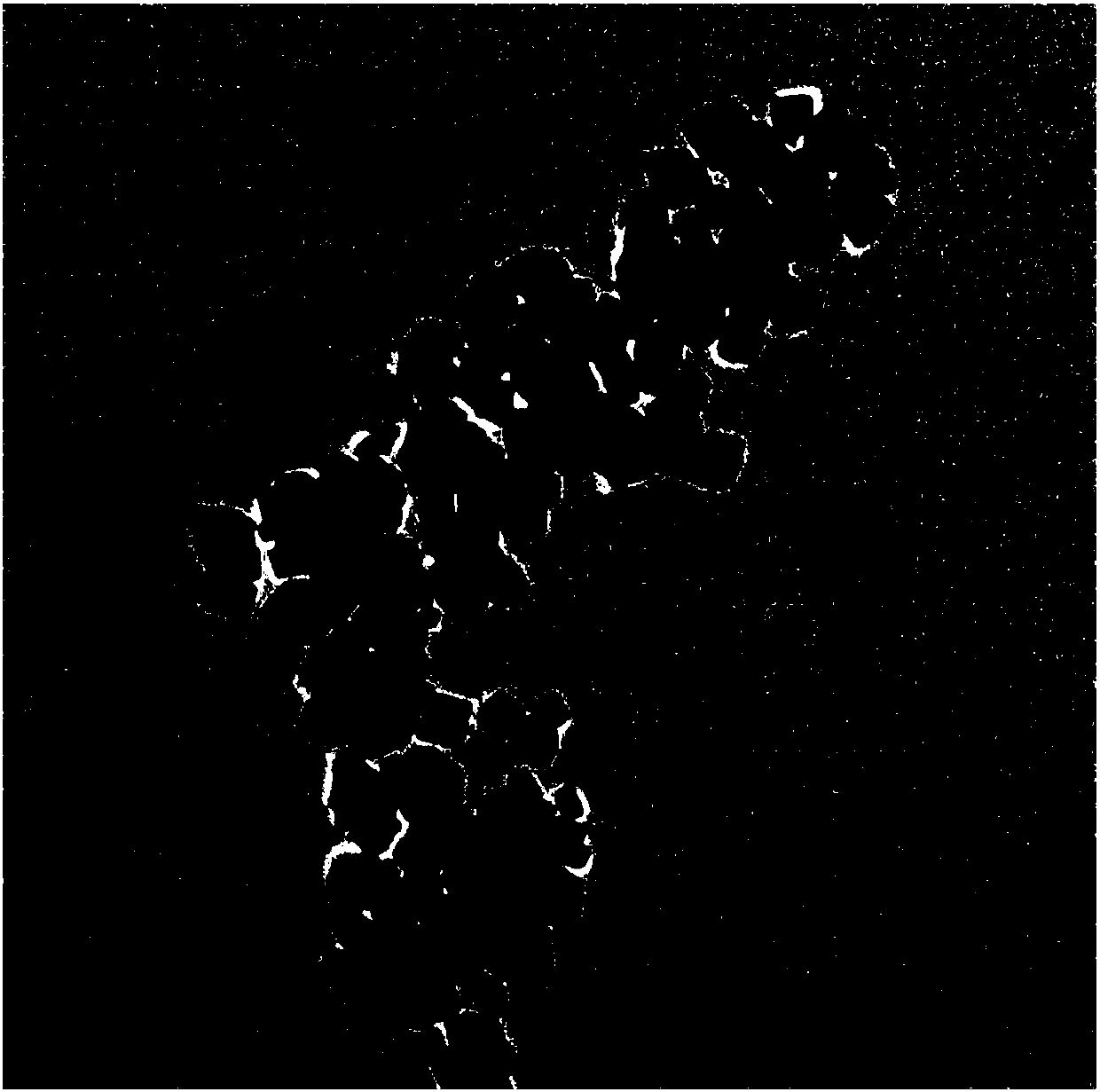
[0037] The lye obtained by dissolving ferric chloride solution with a concentration of 0.13mol / L and 2g of sodium hydroxide in a mixed solvent composed of 25mL of water, 50mL of oleic acid and 50mL of ethanol is pumped into the supergravity rotating packed bed at the same time through a delivery pump. The feed flow ratio is 3:5, and the rotation speed is set at 1000rpm to obtain a precursor suspension with uniform crystal nuclei; transfer the precursor suspension to a 250mL hydrothermal kettle, and crystallize in an oven at 180°C for 10h; After the crystallization reaction is completed, after the hydrothermal kettle is cooled to room temperature, the product is centrifuged to obtain a reddish-brown precipitate at a centrifugal speed of 5000 rpm, and washed with ethanol for 4 to 5 times; ℃ drying for 8 hours; cooling to room temperature and grinding to obtain nano-α-Fe 2 o 3 Powder. figure 2 It shows that the particle shape of the product prepared based on Example 1 is cubic...
Embodiment 2
[0039] The lye obtained by dissolving ferric chloride solution with a concentration of 0.13mol / L and 2g of sodium hydroxide in a mixed solvent composed of 25mL of water, 50mL of oleic acid and 50mL of ethanol is pumped into the supergravity rotating packed bed at the same time through a delivery pump. The feed flow ratio is 3:5, and the rotation speed is set at 2000rpm to obtain a precursor suspension with uniform crystal nuclei; transfer the precursor suspension to a 250mL hydrothermal kettle, and crystallize in an oven at 180°C for 10h; After the crystallization reaction is completed, after the hydrothermal kettle is cooled to room temperature, the product is centrifuged to obtain a reddish-brown precipitate at a centrifugal speed of 5000 rpm, and washed with ethanol for 4 to 5 times; ℃ drying for 8 hours; cooling to room temperature and grinding to obtain nano-α-Fe 2 o 3 Powder. image 3 It shows that the particle shape of the product prepared based on Example 2 is cubic,...
Embodiment 3
[0041] The lye obtained by dissolving ferric chloride solution with a concentration of 0.13mol / L and 2g of sodium hydroxide in a mixed solvent composed of 25mL of water, 50mL of oleic acid and 50mL of ethanol is pumped into the supergravity rotating packed bed at the same time through a delivery pump. The feed flow ratio is 3:5, and the rotation speed is set at 2500rpm to obtain a precursor suspension with uniform crystal nuclei; transfer the precursor suspension to a 250mL hydrothermal kettle, and crystallize in an oven at 180°C for 10h; After the crystallization reaction is completed, after the hydrothermal kettle is cooled to room temperature, the product is centrifuged to obtain a reddish-brown precipitate at a centrifugal speed of 5000 rpm, and washed with ethanol for 4 to 5 times; ℃ drying for 8 hours; cooling to room temperature and grinding to obtain nano-α-Fe 2 o 3 Powder. Figure 4 It shows that the particle shape of the product prepared based on Example 3 is cubic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com