Glucose dehydrogenase dna molecule, carrier and bacterial strain and application
A technology of glucose dehydrogenase and DNA molecules, which is used in the construction of mutants and engineering bacteria, the production of DNA molecules, proteins or polypeptide sequences of glucose dehydrogenase. stability issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0101] Embodiment 1 provides a kind of route of constructing the mutant gene of the present invention, as follows:
[0102] (1) Screening of mutant genes
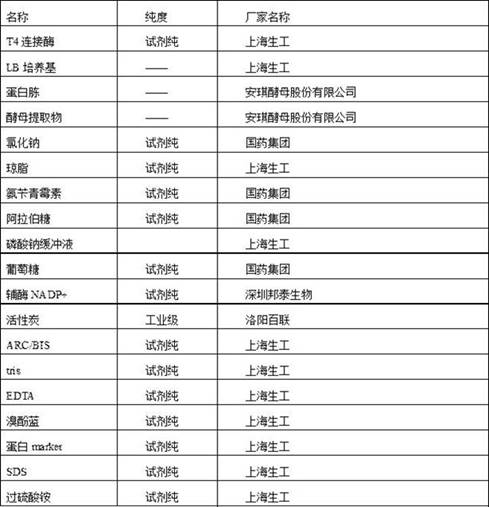
[0103] Using the keyword "glucose dehydrogenase" to search on NCBI, after comparative analysis, the glucose dehydrogenase (GDH) gene sequence in Bacillus subtilis subsp. Subtilis str.AG1839 was selected as the research template, in which Bacillus subtilis The sequence of the glucose dehydrogenase of subsp. Subtilis str.AG1839 is SEQ ID NO.3,
[0104]Wherein, the sequence of SEQ ID NO.3 is as follows:
[0105] atgtatccgg atttaaaagg aaaagtcgtc gctattacag gagctgcttc agggctcgga 60
[0106] aaggcgatgg ccattcgctt cggcaaggag caggcaaaag tggttatcaa ctattatagt 120
[0107] aataaacaag atccgaacga ggtaaaagaa gaggtcatca aggcgggcgg tgaagctgtt 180
[0108] gtcgtccaag gagatgtcac gaaagaggaa gatgtaaaaa atatcgtgca aacggcaatt 240
[0109] aaggagttcg gcacactcga tattatgatt aataatgccg gtcttgaaaa tcctgtgcca 300
[0110] tctcacgaaa tgccgctcaa ...
Embodiment 2
[0182] Example 2 provides a comparison experiment of the specific enzyme activity of the glucose dehydrogenase produced by the strain prepared in Example 1 of the present invention and the glucose dehydrogenase produced by the original strain.
[0183] Among them, the specific enzyme activity is the number of enzyme activity units per mg per protein, that is, the enzyme activity units are divided by the mass of the enzyme protein.
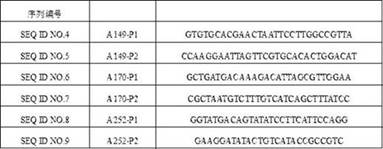
[0184] The target fragment gene of known sequence and the gene fragment of glucose dehydrogenase and the 149-170 double-mutated mutant were respectively connected to the large fragment of plasmid pBAD, and 10 μL of the ligation reaction was added to 100 μL of competent bacteria. Gently mix in the EP tube, ice bath for 30min, heat shock at 42°C for 90s, take it out and quickly ice bath for 2min to cool the bacteria. Add 1 mL of LB liquid medium without antibiotics (containing 10 μL of arabinose inducer), shake at 200 rpm for 1 h at 37 °C. Take 100 ...
Embodiment 3
[0188] Embodiment 3 provides the application of the glucose dehydrogenase prepared in Embodiment 1 in the field of biosynthesis.
[0189]
[0190] Formula (Ⅱ)
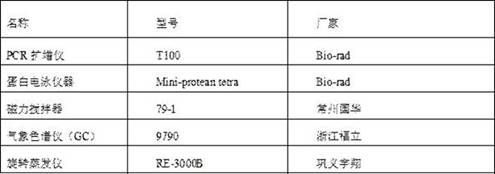
[0191] Add 2.4kg substrate (S)-6-chloro-5-hydroxy-3-oxohexanoic acid tert-butyl ester (67% concentration), 25kg sodium phosphate buffer, 2kg glucose solid, 2kg Glucose dehydrogenase solution (2500U / ml), 9kg ketoreductase (80U / ml) and 500mg coenzyme NADP+, magnetic stirring, reaction temperature 30°C, pH=7.0. Among them, the gas phase detection conditions are: chromatographic column type, HP-5, injection volume 1 μL; injector temperature, 270°C; column oven temperature, programmed temperature rise of 100°C for 3 minutes, then 10°C / min to 220°C; FID Detector temperature, 270°C. The gas phase detection conditions were used to detect the completeness of the reaction.
[0192] Then add 300g of activated carbon to the reaction system and stir for 30min, then filter, the filtrate is extracted twice with 40L and 20L of e...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com