Marine bacterium derived thermal-stable and salt-tolerant SGNH family hydrolytic enzyme and application
A hydrolytic enzyme, consistent technology, applied in the field of genetic engineering, can solve problems such as unclear function
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1 Acquisition of hydrolase gene aline4
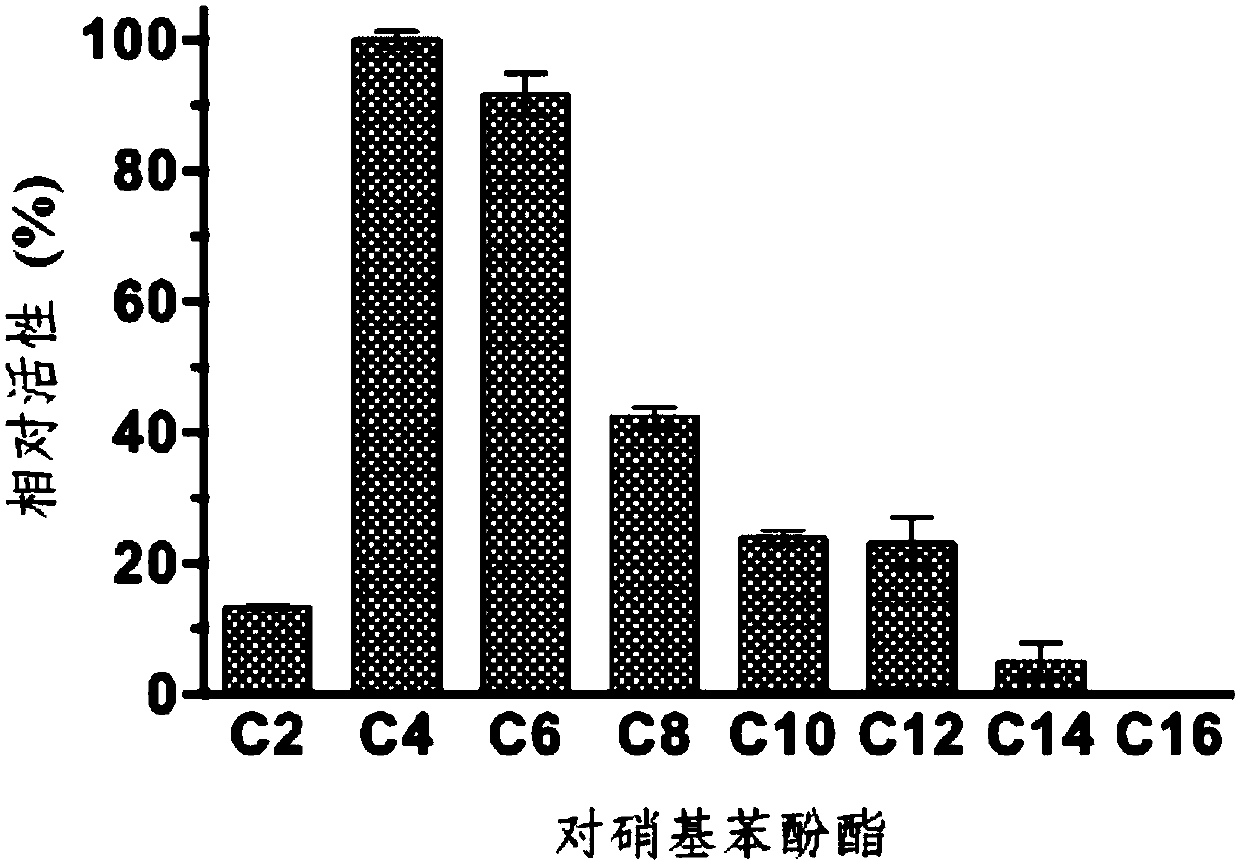
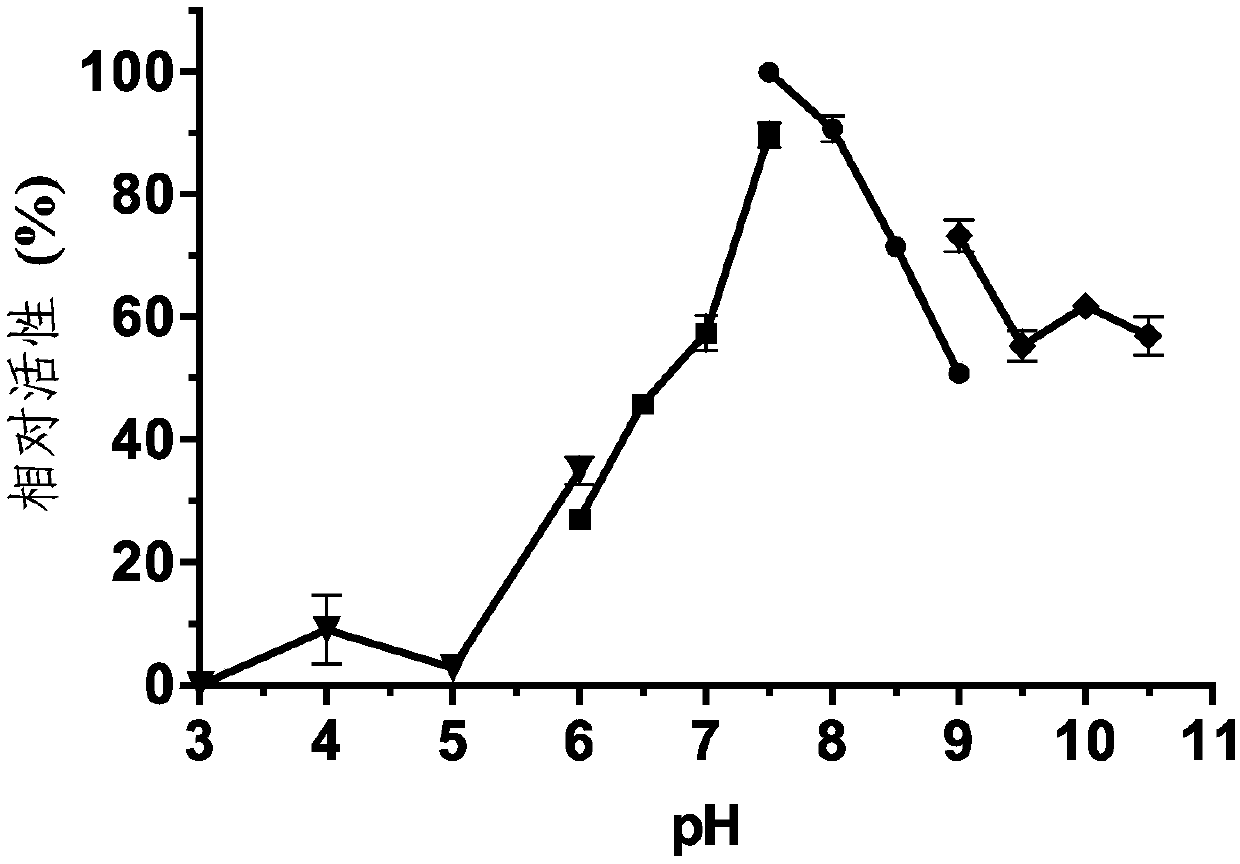
[0042] Based on the complete genome, open reading frame prediction and gene annotation results of the bacteria Altererythrobacter indicus DSM18604 isolated from the mangrove wild rice nodule soil, the lipohydrolase-related genes were screened. The homology between the sequences and the known hydrolase gene sequences in the database was compared by Blastp (http: / / blast.ncbi.nlm.nih.gov / ). The aline4 gene was obtained through database comparison analysis, with a size of 573bp and a base composition of 139A (24.26%), 115T (20.07%), 149C (26.00%) and 170G (29.67%), and its nucleotide sequence is as shown in SEQ ID No :1 shown. The size of the encoded protein is 190 amino acid residues, and its amino acid sequence is shown in SEQ ID No.2. Homology search was performed on the AlinE4 protein sequence in GenBank, and the highest amino acid sequence identity with it was non-cultured source esterase, the identity was 71%, and its...
Embodiment 2
[0045] Construction of the recombinant expression plasmid and recombinant strain of embodiment 2 gene aline4
[0046] The gene aline4 obtained in the present invention is cloned into an expression vector to construct a recombinant expression strain. Based on the gene open reading frame sequence obtained by the ORF analysis of NCBIORF Finder, the upstream primer aline4F (5'-TCGC GGATCC ATGGGCGAATCGCGC-3', BamHI) and downstream primer aline4R (5'-TCCG CTCGAG TCACTTCTTCGCAGGCAGCGCC-3', XhoI), PCR amplification confirms the full-length sequence of the gene. The expression plasmid was constructed by enzyme digestion cloning, that is, the PCR product was double-digested with BamHI and XhoI, and the purified fragment was ligated with the plasmid pSMT3 that had been double-digested with BamHI and XhoI. 2 The transformation method was transformed into E.coli DH5α, and positive clones were screened for kanamycin resistance. A plasmid extraction kit (Omega, USA) was used to extract ...
Embodiment 3
[0047] Example 3 Utilize the recombinant expression strain to express the recombinant gene aline4
[0048] Transfer 3ml of the constructed recombinant expression strain to 100ml of LB liquid medium containing 20μg / ml kanamycin and 34μg / ml chloramphenicol, and shake at 37°C until OD 600 When it reaches 0.6, add IPTG with a final concentration of 0.5mM to induce expression, transfer to 20°C and shake at 150r / min for 16h. The bacterial cells were collected by low-temperature centrifugation, resuspended in NTA-10 solution (500 mM sodium chloride, 10 mM imidazole, 20 mM Tris hydrochloric acid, pH 8.0), and subjected to sonication on ice. Centrifuge at low temperature to collect supernatant, use NTA-Ni 2+The expressed protein was purified by affinity column chromatography. The expressed recombinant protein contains a 6×His tag at the N-terminal, which can be affinity-adsorbed to the layer suction column, and is eluted through gradient elution with different concentrations of imida...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com