Local delivery eye medicine composition
A composition and ophthalmic technology, applied in the field of medicine, can solve the problems of serious adverse reactions, blindness, dissimilarity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
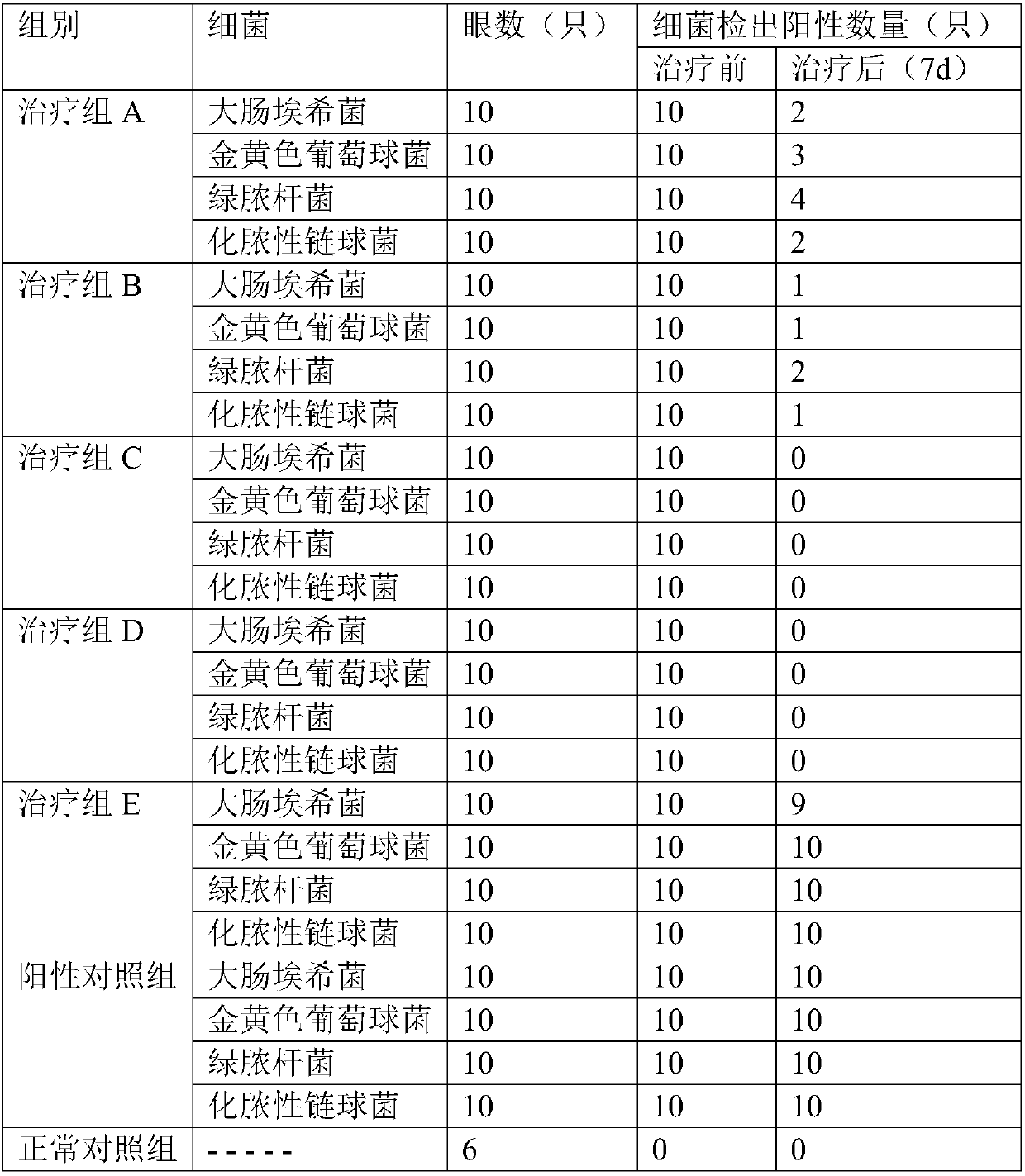
Image
Examples
preparation example Construction
[0104] In the preparation process of each embodiment of the present invention, in the case where the prescription of the embodiment has defined the names of the components, for the sake of simplicity, the names of the components in the prescription can be simplified or omitted.
[0105] Concentration units used in the description of the present invention have molar concentration (M) or normal concentration (N), or percentage concentration etc., time unit can use second (s), minute (min), hour (h) etc., volume unit can use Liter (l or L), milliliter (ml), microliter (μl), mass unit can be gram (g), milligram (mg) and so on.
Embodiment 1
[0108] The preparation of embodiment 1 etimicin sulfate eye drops
[0109]
[0110] Preparation process: take the prescribed amount of sodium hyaluronate, add it to an appropriate amount of water for injection, heat, dissolve and let it cool, and dissolve etimicin sulfate in the water for injection, and then add the prescribed amount of boric acid, borax, taurine, benzene For ammonium chloride, stir to dissolve, then add sodium hyaluronate solution to the solution, stir, adjust the pH value to 6.2 with citric acid solution and sodium hydroxide solution, add water to the full amount, pressurize through a 0.45 μm microporous membrane Filter, after passing the test, fill in 5ml / bottle, seal, sterilize at 121°C, let cool, inspect, and pack.
[0111] Take 10 bottles of the above samples and place them away from light for 6 months at a room temperature of about 25°C and a relative humidity of 75%±5%. The solution remains clear and the pH value of the solution is measured to be 6....
Embodiment 2
[0112] The preparation of embodiment 2 etimicin sulfate eye drops
[0113]
[0114] Preparation process: Take the prescribed amount of sodium hyaluronate, add it to an appropriate amount of water for injection, heat it to dissolve and let it cool, take another etimicin sulfate and dissolve it in the water for injection, add the prescribed amount of sodium dihydrogen phosphate and disodium hydrogen phosphate in turn , ethylparaben, sodium chloride, nicotinamide, stir to dissolve, then add sodium hyaluronate solution to the solution, stir, adjust the pH value to about 6.3 with lactic acid solution and sodium citrate solution, add water to the full amount , filtered through a 0.45 μm microporous membrane under pressure, after passing the test, fill in 8ml / bottle, seal, sterilize at 121°C, let cool, inspect, and pack.
[0115] Take 10 bottles of the above-mentioned samples and place them away from light for 6 months at a room temperature of about 25°C and a relative humidity of...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com