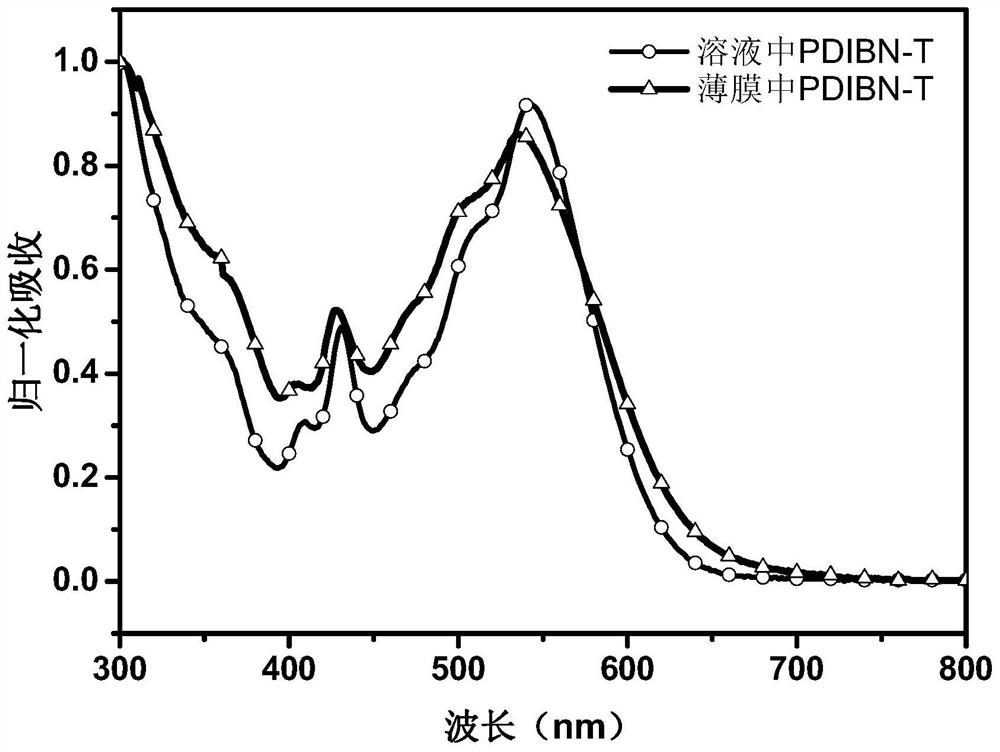
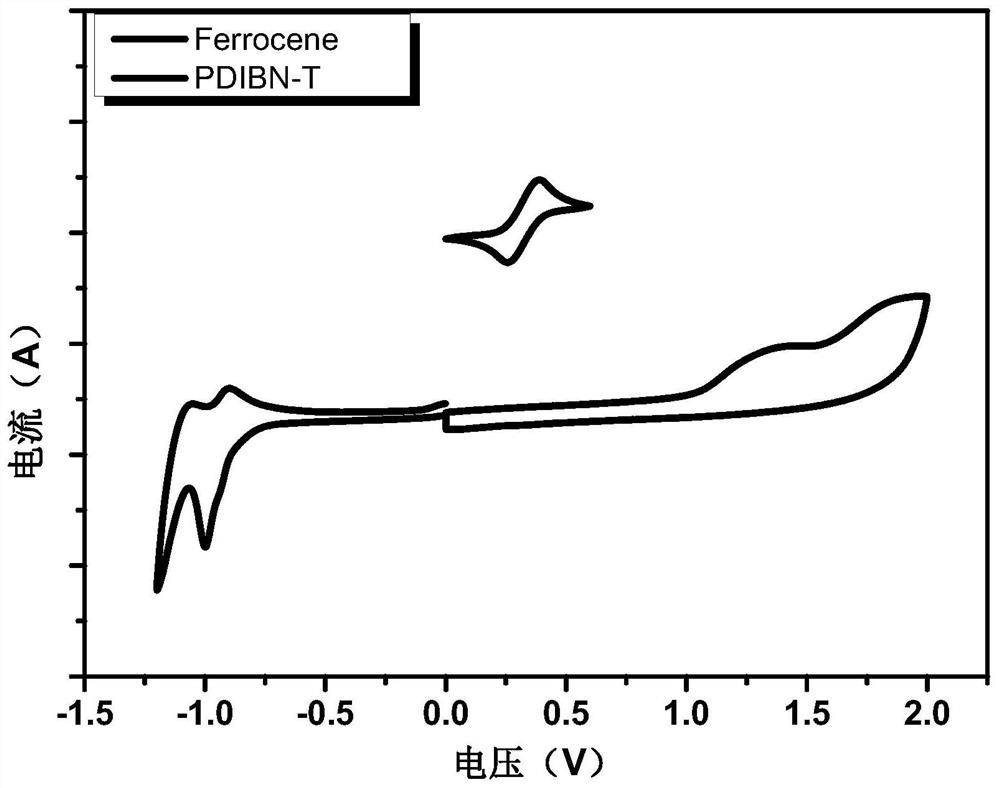
A kind of boron-nitrogen bond-containing perylene imide optoelectronic small molecule material and its preparation method and application
A perylene imide and small molecule technology, which is applied in the field of perylene imide photoelectric small molecule materials and its preparation, can solve the problems of less research on electron transport materials, achieve broad application prospects, short process flow, high electronic and The effect of hole mobility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The preparation of peryleneimide photoelectric small molecule material PDIBN-T containing boron nitrogen bond, the reaction equation is as follows:
[0040]
[0041] (1) In a 500mL three-neck flask, under nitrogen protection, add compound M1 (856mg, 0.1mmol) and propylamine (59mg, 0.1mmol), then add 15mL tetrahydrofuran as a solvent, react at room temperature for 12 hours, and determine by spotting Reaction process; after the reaction, the reaction mixture was put into a one-necked bottle for direct rotary evaporation, and purified by column chromatography (dichloromethane) to obtain 700 mg of a yellow-orange dark green solid, namely compound M2, with a yield of 80%;
[0042] (2) In a 500mL three-necked flask, under nitrogen protection, add compound M2 (292mg, 0.35mmol) and dichloro(phenyl)borane (0.4ml); then add 1mL of catalyst triethylamine and 30mL of Toluene, the solvent, started to heat up after the dropwise addition, and heated to 100°C for 12 hours of reactio...
Embodiment 2
[0050]
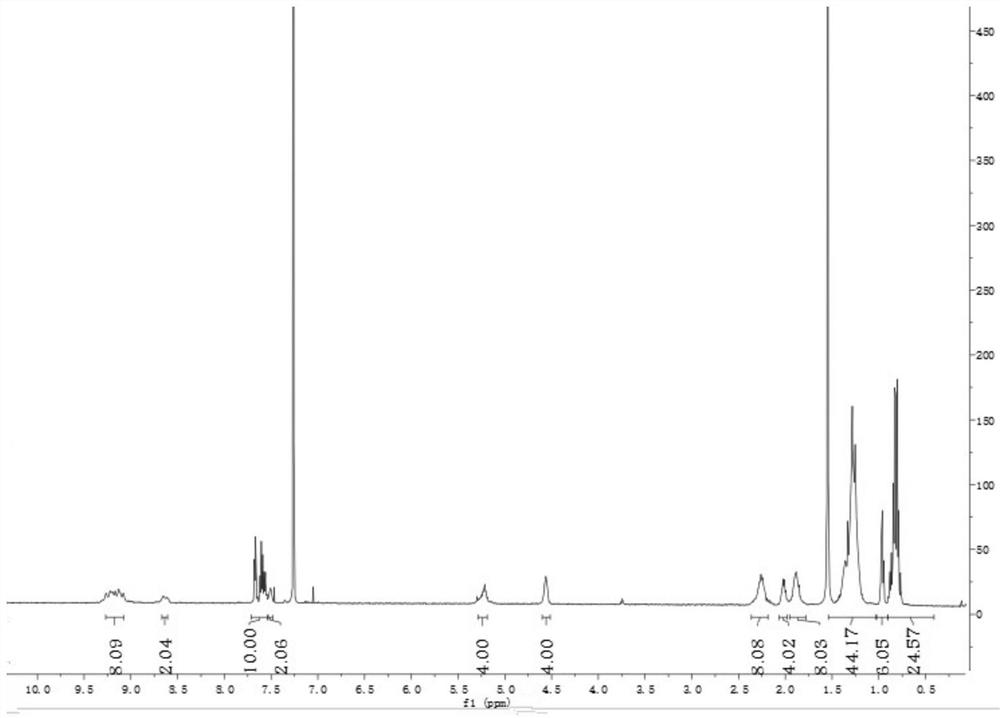
[0051] Under nitrogen protection, in a 500mL three-necked flask, add compound M3 (230mg, 0.25mmol) and 5,5'-bis(trimethyltin)-2,2'-dithiophene (58mg, 0.12mmol), and then Toluene (10 mL) was added as a solvent and tetrakis(triphenylphosphine) palladium (9 mg) was added as a catalyst. After the addition, the temperature began to rise, and the mixture was heated to 130° C. for 12 hours. After the reaction, extract with dichloromethane (DCM), while stirring silica gel powder, the crude product is purified with silica gel column (petroleum ether:dichloromethane volume ratio is 2:5). Finally, recrystallization was carried out with methanol and tetrahydrofuran to finally obtain 216 mg of dark red solid, which is the small molecule material PDIBN-DT, with a yield of 72%. 1 H NMR: (500MHz, CDCl 3 )δ9.27–9.08(m,8H),8.65(s,2H),7.71–7.54(m,10H),7.51(s,2H),7.32(s,2H),5.22(s,4H),4.56 (s,4H),2.25(d,J=8.6 Hz,8H),2.07–1.98(m,4H),1.87(d,J=14.0Hz,8H),1.54–1.03(m,44H),0.96( t, J=7....
Embodiment 3
[0053] Fabrication of battery devices
[0054] With PBDB-T (poly[[4,8-bis[5-(2-ethylhexyl)-2-thienyl]benzo[1,2-b:4,5-b']dithiophene-2, 6-diyl]-2,5-thiophenediyl[5,7-bis(2-ethylhexyl)-4,8-dioxo-4H,8H-benzo[1,2-c:4, 5-b'] dithiophene-1,3-diyl]]) and the small molecule material PDIBN-T prepared in Example 1 are blended as the battery active layer material to make an organic solar cell, and the active layer material donor PBDB- The ratio of T to acceptor PDIBN-T was 1:1.5 (w / w).
[0055] Formal device: ITO glass (indium tin oxide conductive glass) is treated with oxygen-Plasma after ultrasonic cleaning, and PEDOT:PSS (polyethylene dioxythiophene) film (thickness 40nm) is used as a hole transport layer on ITO , and then use PBDB-T and the PDIBN-T blend solution prepared in Example 1 to spin the film, which is the active layer (thickness 100nm), and anneal the active layer at 100° C. for 5 minutes. A layer of PFN (poly[9,9dioctylfluorene-9,9-bis(N,N dimethylamino-hexyl)fluorene])...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com