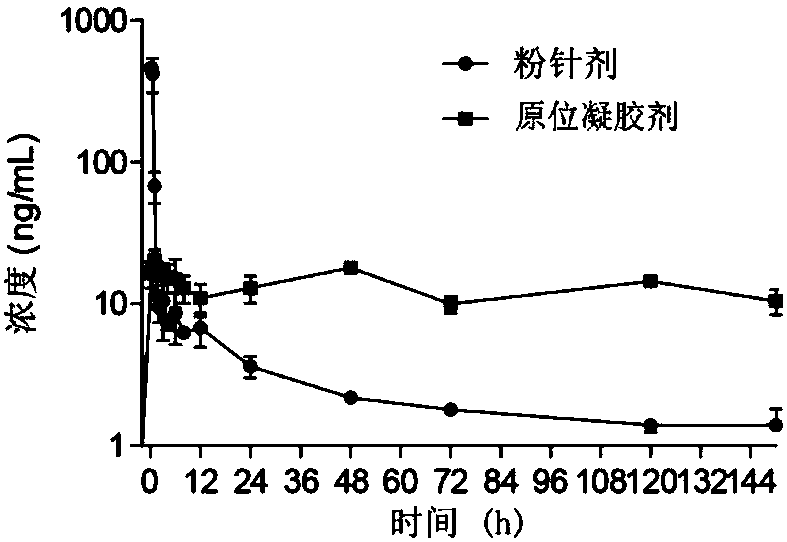
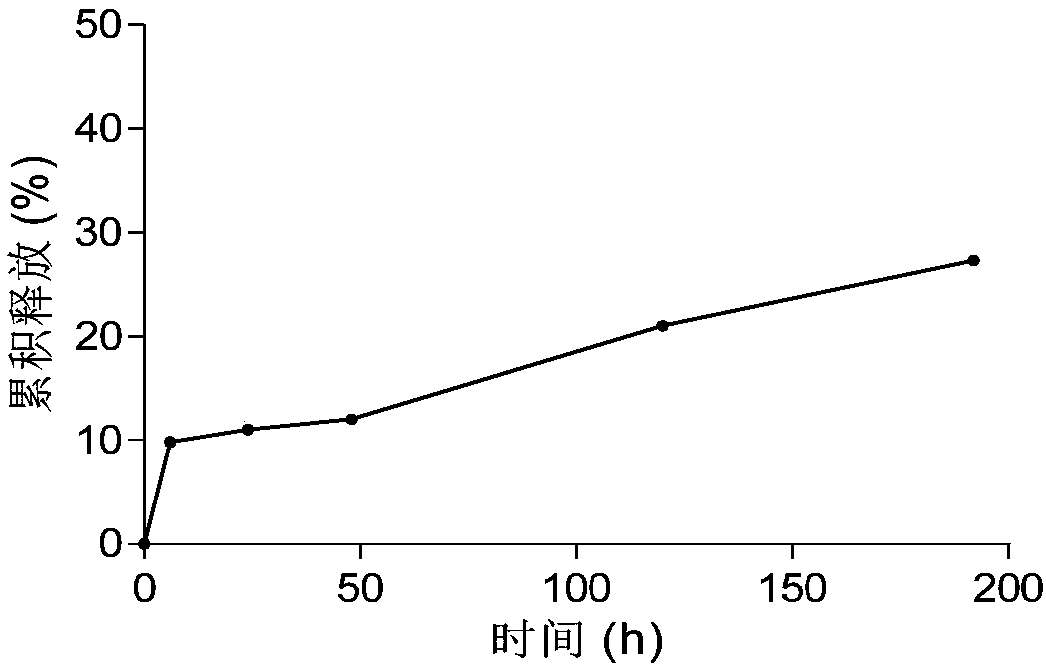
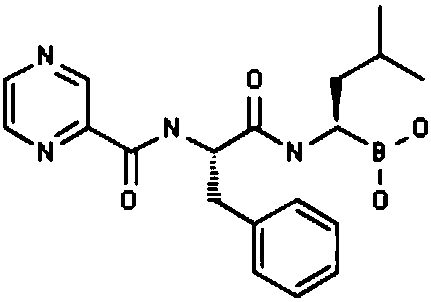
Bortezomib pharmaceutical composition and applications thereof
A technology of bortezomib and its composition, which is applied in the field of pharmaceutical preparations, can solve the problems of poor compliance of patients, and achieve the effects of long-lasting drug effect, precise blood drug concentration, and stable proteasome inhibition level for a long time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The preparation method of the multivesicular liposome of the present invention adopts conventional methods in the art, such as adopting the double emulsion method, specifically, it needs to include the following 5 steps: (1) first dissolve the lipid component of the prescription amount in easy The volatilized organic solvent (usually the mixed solution of chloroform or chloroform and ether) forms an oil phase, and the bortezomib of the recipe quantity is dissolved in water to form a drug-containing aqueous solution (the first water phase), and then with a suitable oil-water volume ratio ( The volume ratio is 1:10-12:10, v / v) by mixing the aqueous solution containing the drug (the first water phase) with the organic phase of the lipid (oil phase), and ultrasonically or mechanically shearing at room temperature for a certain period of time to prepare Uniform water-in-oil (W / O) type colostrum; (2) absorb the formed W / O type colostrum, and inject the second aqueous phase buf...
Embodiment 1
[0079] The preparation of embodiment 1 bortezomib in situ gel preparation (reservoir type preparation)
[0080]
[0081] After dissolving and dispersing the PEGylated PLA with N-methylpyrrolidone, the drug bortezomib is dissolved to obtain the in situ gel injection.
Embodiment 2
[0087] Embodiment 2: Preparation of bortezomib microspheres
[0088]
[0089] Dissolve bortezomib and PLGA in dichloromethane, and inject it into BUCHI B-290 with a drying temperature of 65°C, a spray frequency of 120kHz, and a ventilation rate of 70L / min at an injection rate of 0.2ml / min at a rate of 0.5ml / min. Dry the electrostatic collection system to prepare microspheres with uniform particle size, and 80% of the microspheres have a particle size of 0.5-10um.
[0090] The release test of the bortezomib microsphere of test embodiment 2 embodiment 2
[0091] The bortezomib microspheres of Example 2 were placed in the release medium of physiological isotonic PBS solution (pH 7.4) and incubated at 37°C, under the condition of 100r / min, at a predetermined time point, 5ml of the dissolution medium was taken, and centrifuged at 10000rpm for 5min , Precisely measure 20 μl of the supernatant and inject the filtrate into the liquid chromatograph, record the chromatogram, and mak...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com