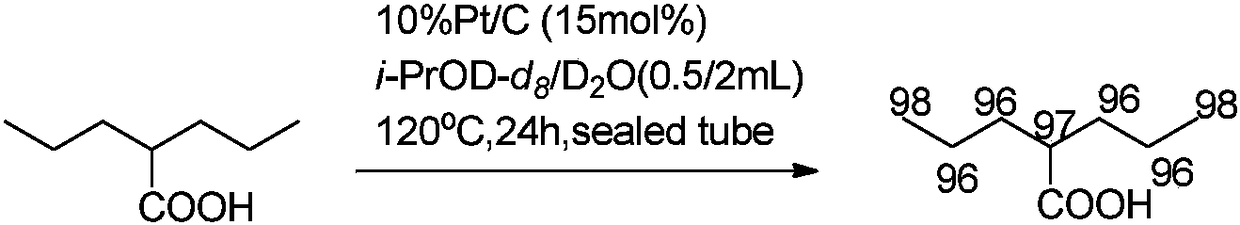
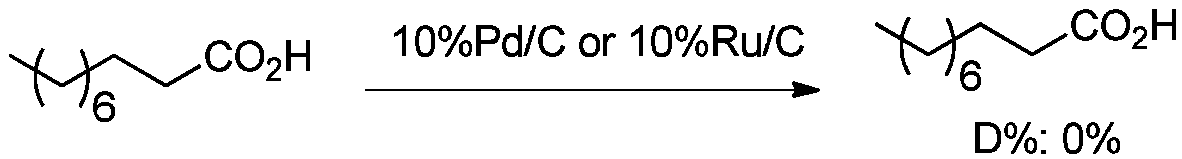Beta-deuterated valproic acid preparation method
A deuterated valproic acid and deuterated technology, which is applied in the field of preparation of antiepileptic drug beta-deuterated valproic acid, can solve the problems of high price of reducing agent deuterated lithium aluminum hydride, no experimental operation, harsh reaction conditions, etc. , to achieve the effect of high yield, low environmental pollution and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation method of embodiment 1, β-deuterated valproic acid comprises the following steps successively:
[0056] (1) Directed deuteration reaction:
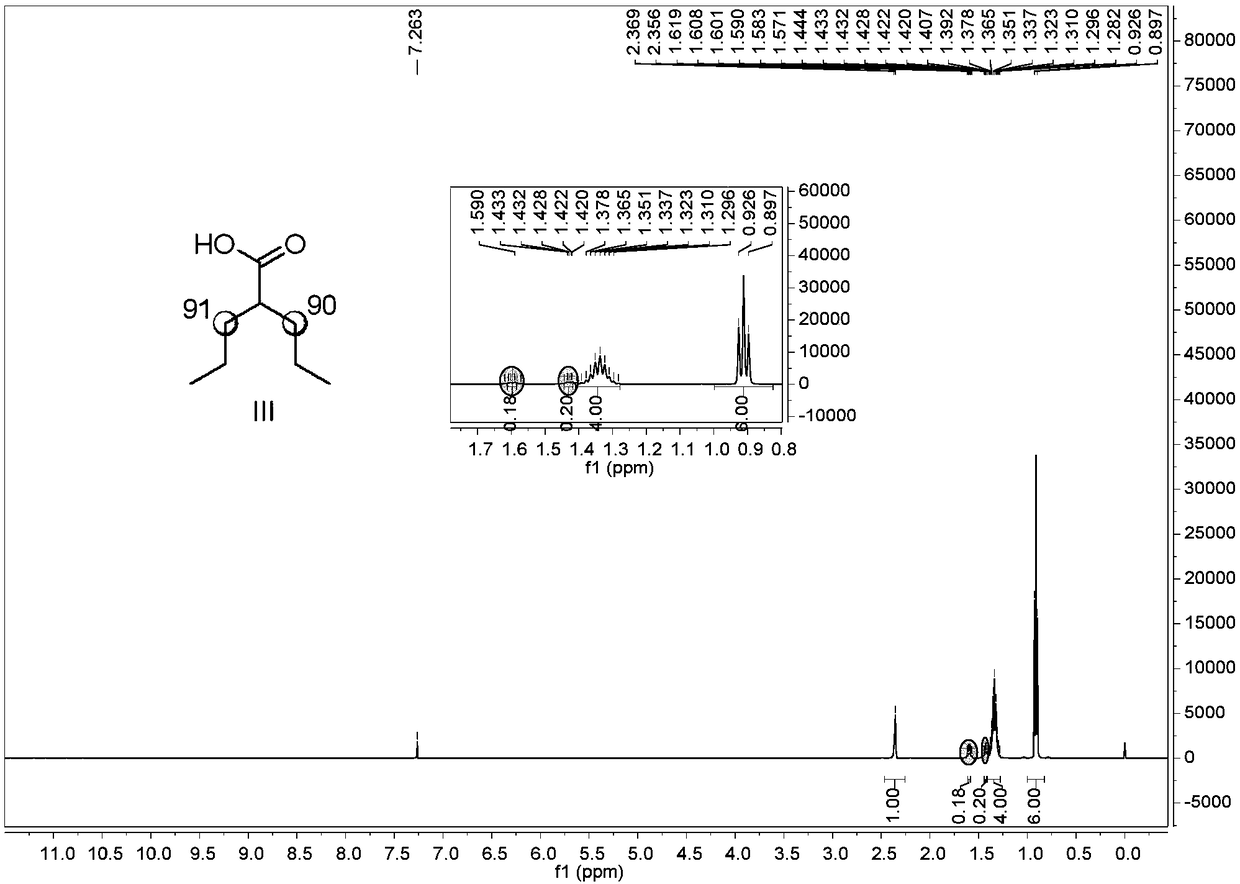
[0057] Weigh 4.13g (15.26mmol, 1eq.) of 8-aminoquinoline valproic acid amide and 0.68g (3.05mmol, 0.2eq.) of palladium acetate into a sealed tube, add 8.3ml of heavy water, and seal the tube at 140°C State reaction 48h.
[0058] After the reaction, add 20ml of water, then add ethyl acetate (3 × 30ml) for extraction, combine the organic phase - ethyl acetate layer, wash with saturated brine (30ml), then dry with 6g of anhydrous sodium sulfate, rotary evaporator Concentrate under reduced pressure, the concentration temperature is 30-40°C, the vacuum degree is not lower than -0.07MPa, concentrate until there is no obvious drop, and the concentrate is separated and purified by silica gel chromatography (the eluent is PE:EA=15:1 ), the intermediate product deuterated 8-aminoquinoline valproic acid amide 3.97g was obtai...
Embodiment 2
[0078] Embodiment 2, repeated application
[0079] Weigh the 8-aminoquinoline (115mg, 0.80mmol) and N,N-dimethyl-4-aminopyridine (13mg, 0.1mmol) recovered in Example 1 into a three-necked reaction flask, and add Anhydrous dichloromethane (10 mL), and triethylamine (135 μL, 0.96 mmol, 1.2 equiv) were added, the reaction system was cooled to 0° C., and valproyl chloride (130 mg, 0.80 mmol) was added dropwise. The reaction system was stirred overnight at room temperature. Add 20 mL of water to quench the reaction, extract with dichloromethane (3×20 mL), combine the dichloromethane layers, dry over anhydrous sodium sulfate, filter with suction, concentrate, and separate and purify by silica gel chromatography (petroleum ether: ethyl acetate=15: 1) 195 mg of 8-aminoquinoline valproic acid amide was obtained as a white solid, with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com