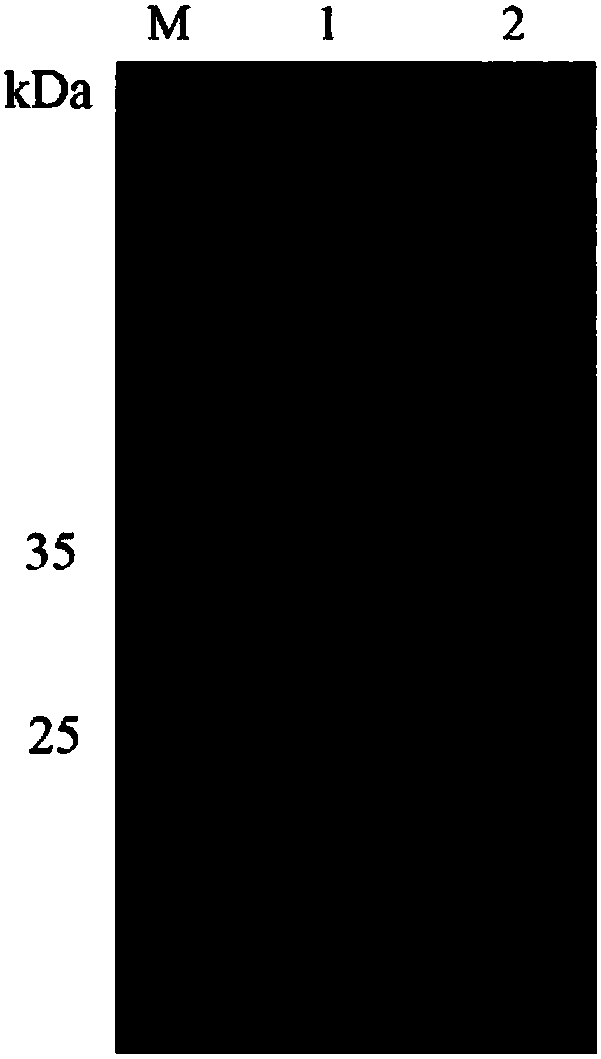
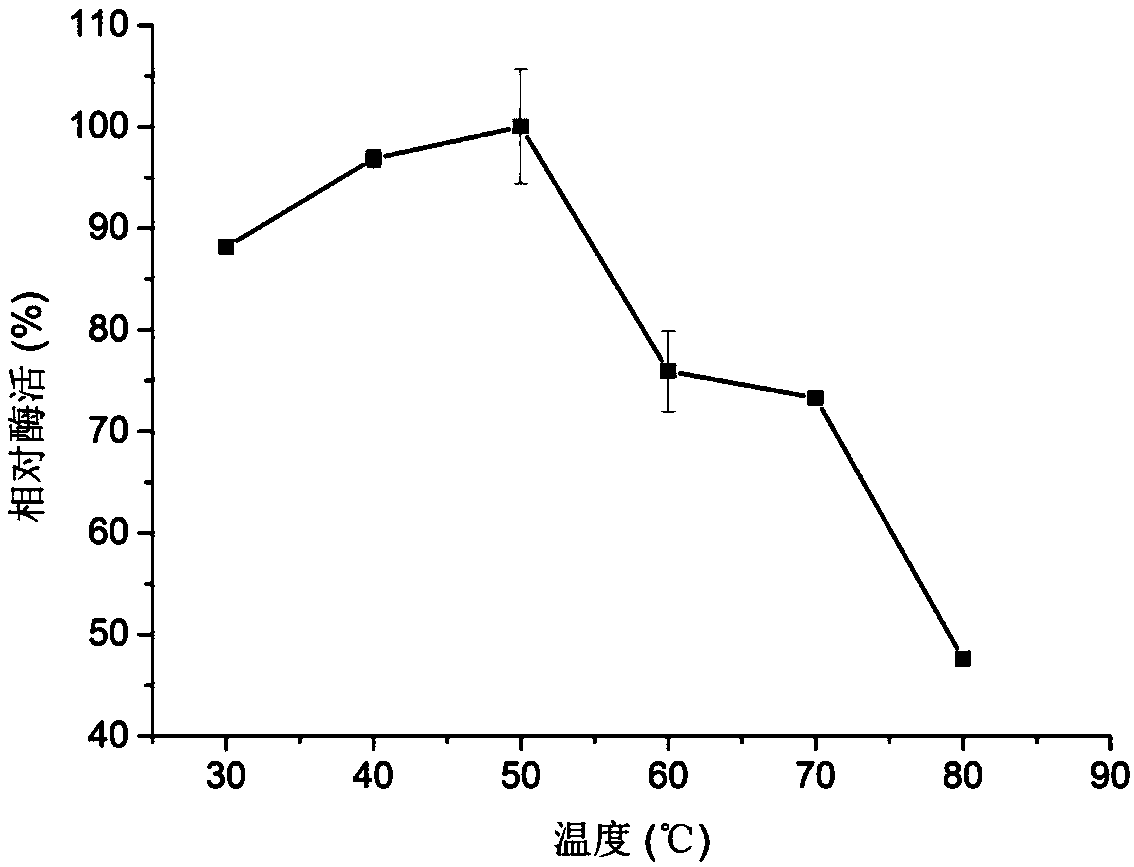
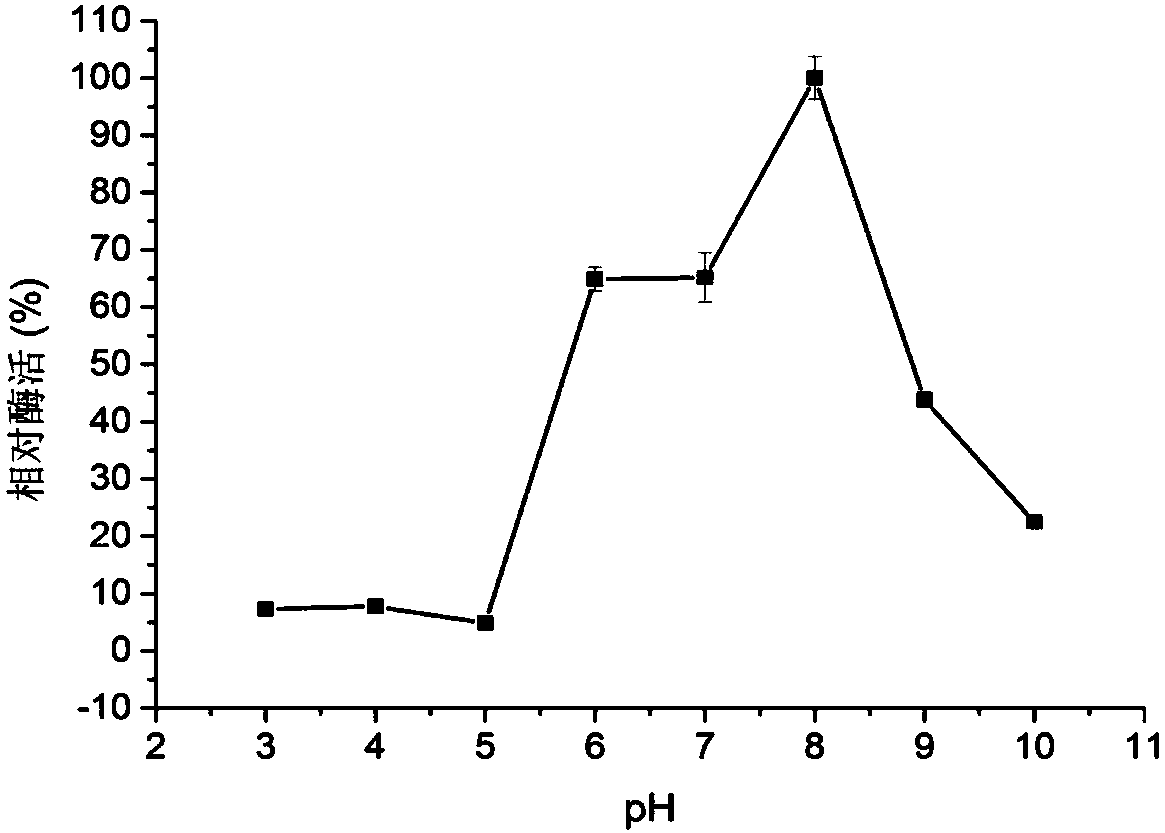
Chitoanase and application thereof to preparation of chitosan oligosaccharide
A technology of chitosan enzyme and chitosan, applied in the direction of application, glycosylase, enzyme, etc., can solve the problems of low chitosan specificity, poor product quality, high equipment requirements, etc., and achieve efficient degradation and good temperature High stability and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 produces the cloning of chitosanase gene fragment
[0018] The applicant analyzed the Streptomyces albolongus genome by molecular biology software, and found that there was a novel chitosanase gene, and by cloning the gene, the present invention was facilitated.
[0019] Using the genome of Streptomyces albolongus as a template, the upstream primer Csn-F 5'-CGCGGATCCATGGGCGCCGGCCGGGCC-3' and the downstream primer Csn-R respectively containing BamHI and HindIII restriction sites were designed on the upstream and downstream of the chitosanase gene 5'-CCCAAGCTTGTCGTACGGGTAGTGACG-3', the Csn gene fragment was amplified by PCR.
[0020] The PCR reaction system is: 2×PCR Buffer 25 μl, dNTP 5 μl, each primer 1 μl, template (Streptomyces albicans whole genome DNA) 1 μl, KOD Fx DNA polymerase (TOYOBO, KFX-101) 1 μl, add sterile water to a final volume of 50 μl. The reaction conditions of PCR were: pre-denaturation at 94°C for 2 min, denaturation at 98°C for 10 s, ...
Embodiment 2
[0022] Embodiment 2 Contains the expression vector construction of chitosanase gene
[0023] Recover PCR target gene fragments. Add BamHI enzyme and HindIII enzyme to the target gene and plasmid pET-28a, respectively, for double enzyme digestion. The enzyme-cut plasmid was recovered by electrophoresis, and the target gene was purified with a kit (Omega). Ligate the purified target gene fragment with the recovered plasmid.
[0024] After the connection is completed, the heat shock method is used to transform, and the connected system is transferred into DH5α competent cells. Use an LB plate containing ampicillin resistance to screen positive transformants, and use T7 universal primers to perform PCR verification on the clones. The consistency of the results is 100%, which proves that the chitosanase gene sequence has been completely cloned into the expression vector, which is a positive clone, and the recombinant plasmid is named pETC.
Embodiment 3
[0025] Embodiment 3 contains the expression of the recombinant plasmid of chitosanase gene and the construction of engineering bacterium
[0026] Extract the recombinant plasmids from the positive clones with correct sequencing and transform them into the host E.coli BL21 competent cells (the transformation process saves the concentration operation of the bacterial solution), and the positive transformants with ampicillin resistance are the goals of successful cloning Engineering bacteria.
[0027] The chitosanase-producing engineered strain was inoculated into 5 mL of resistant LB medium (100 μg / mL ampicillin resistance), and cultured overnight at 37° C. at 180 rpm for 12 hours to activate the strain. After the bacterial solution was activated, it was added to ZYP-5052 medium (100 μg / mL ampicillin resistance) according to 1% inoculation amount, and cultured at 20° C. and 220 rpm for 48 hours at low temperature to induce the expression of chitosanase.
[0028] After expressin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com