Sacubitril sodium salt, eutectic mixture of sacubitril free acid and acetic acid, crystal form of eutectic mixture, and preparation method and use of crystal form
A technology of sacubitril and co-crystal, applied in the field of pharmaceutical polymorphs, can solve problems such as unfavorable purification, storage, difficult purification, easy moisture absorption, etc., and achieves control of species and content, good thermal stability, and difficult moisture absorption. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
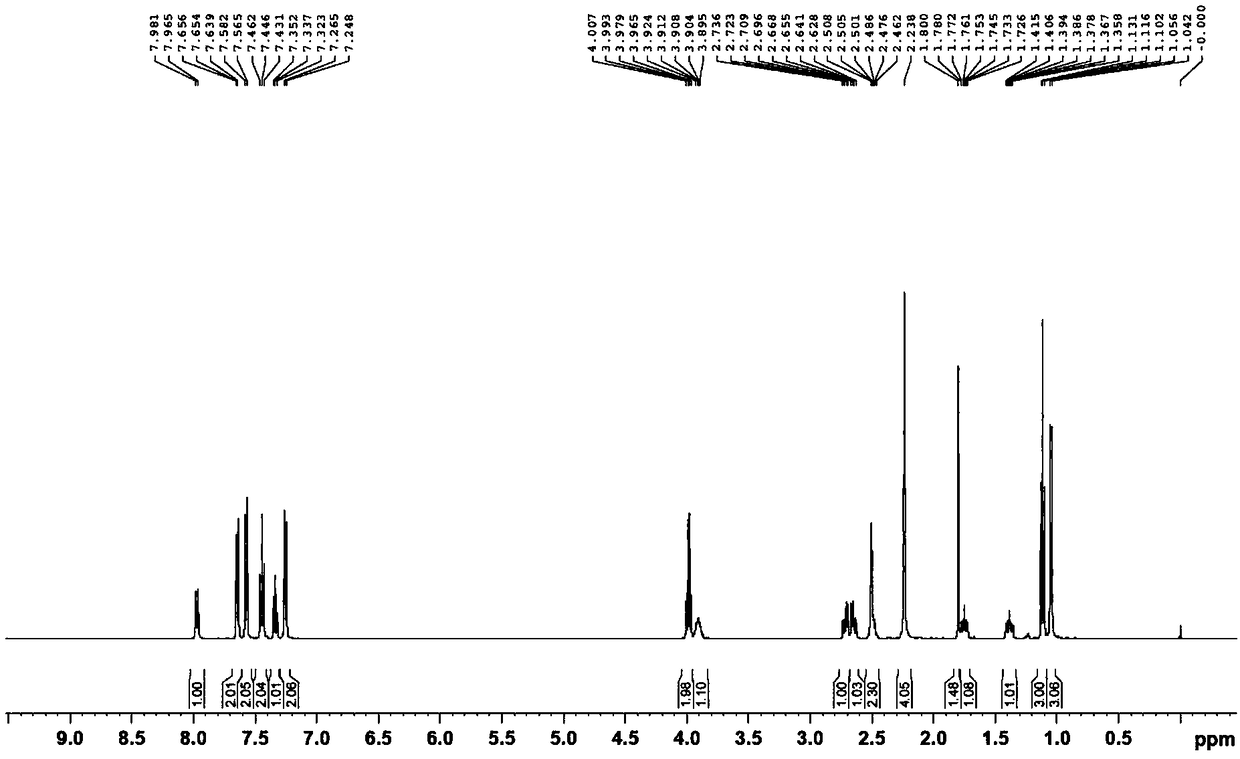
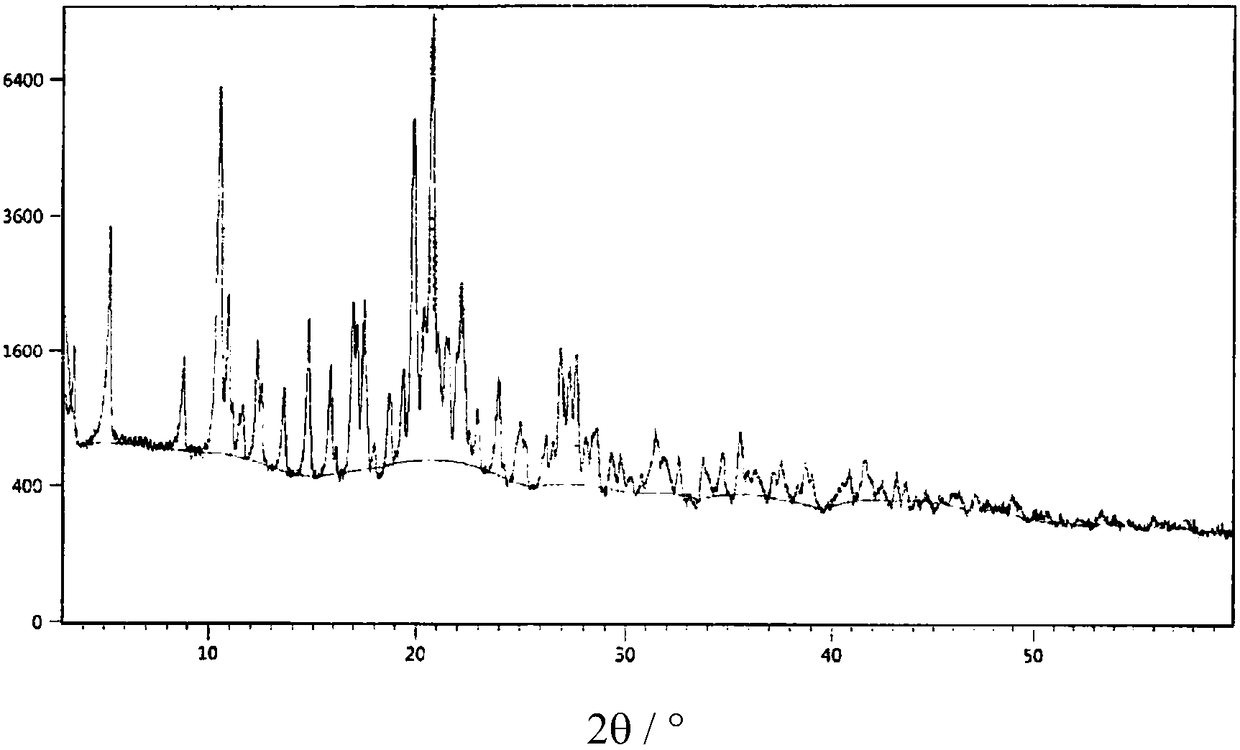
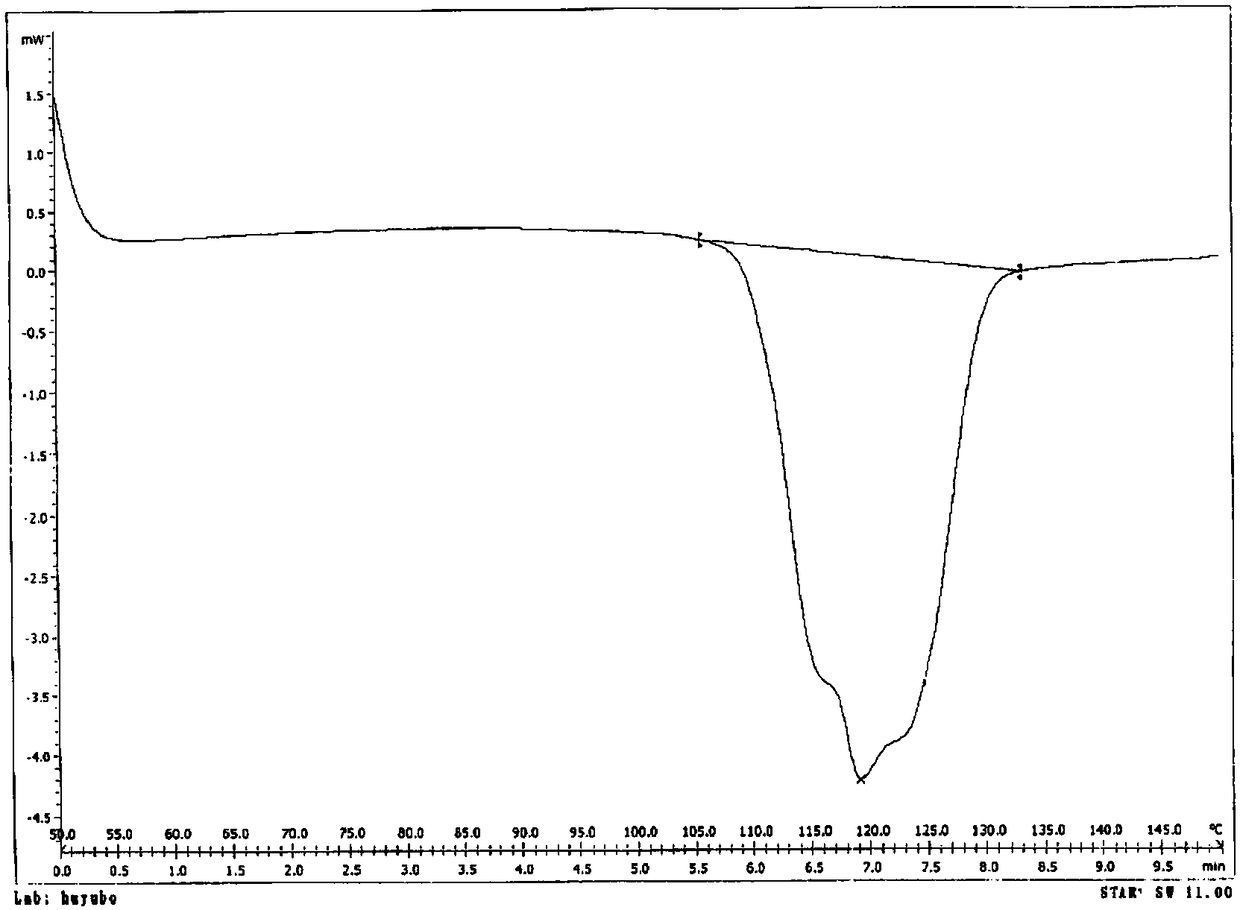
[0058] Example 1: Preparation of cocrystal IV of sacubitril sodium salt, sacubitril free acid and acetic acid
[0059] Add 150mL of dichloromethane and 41.1g of sacubitril free acid oil into the reaction flask, stir to dissolve, then add 9.0g of anhydrous sodium acetate, heat up to 30-35°C and keep the reaction for 1 hour. After the heat preservation is completed, filter. Add 1500mL n-heptane to another reaction flask and cool down to 0-5°C. At this temperature, slowly add the dichloromethane solution obtained from the previous step into the n-heptane dropwise. °C for 1 hour. Filtration, vacuum extraction to obtain 38 g of eutectic IV, yield 83%, chromatographic purity 99.3%.
[0060] Three copies of the eutectic obtained in Example 1 were taken in parallel, and the moisture content of these eutectics was measured by a Karl Fischer moisture meter to be 0.15wt.% to 0.30wt.%, which was far from satisfying that at least one water content was contained in the eutectic. The mass ...
example 2
[0073] Example 2: Preparation of LCZ696 from Cocrystal IV
[0074] The eutectic IV (64g, 142mmol) prepared by the method described in Example 1 was dissolved in a mixed solvent of isopropyl acetate 60mL and acetone 1220mL, heated to 50-55°C and stirred to dissolve, then added valsartan (63g , 145mmol), then dropwise added 142g of aqueous sodium hydroxide solution (10%, 355mmol) at this temperature, then kept at 40~45°C for 2 hours, then cooled to room temperature (25~30°C) for 2 hours. After the heat preservation is completed, the temperature is raised to 40±3°C, 600mL of isopropyl acetate is added dropwise, and the temperature is kept at 40±3°C for 0.5 hours. At the end of the heat preservation, 760mL of solvent was removed under reduced pressure at a temperature of 35±5°C. After the removal is completed, the temperature is raised to 40±3°C, 920 mL of isopropyl acetate is added dropwise, and the temperature is controlled at 40±3°C for 0.5 hours. At the end of the heat prese...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com