A preparation method of cypress biflavone derivatives and its anti-breast cancer application
A kind of cypress biflavonoids, anti-breast cancer technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The separation of embodiment 1 cypress biflavone
[0026] Industrialized countercurrent chromatography (UK Dynamic Extract company) was applied to the separation and purification of cypress biflavonoids from the crude extract of Selaginella chinensis, and the solvent system consisted of 8:8:9:7 n-pentane-ethyl acetate-methanol-water Two-phase system, according to this ratio, the two-phase solvent system is injected into the column through different pumps at a rate of 600mL / min. Obtained cypress biflavone monomer 10g, the whole separation time is only 30min, for the first time broke through the technical difficulties of long separation time and low separation efficiency of countercurrent chromatography technology, and realized the rapid and large-scale preparation of high economic value monomer compound technology by countercurrent chromatography.
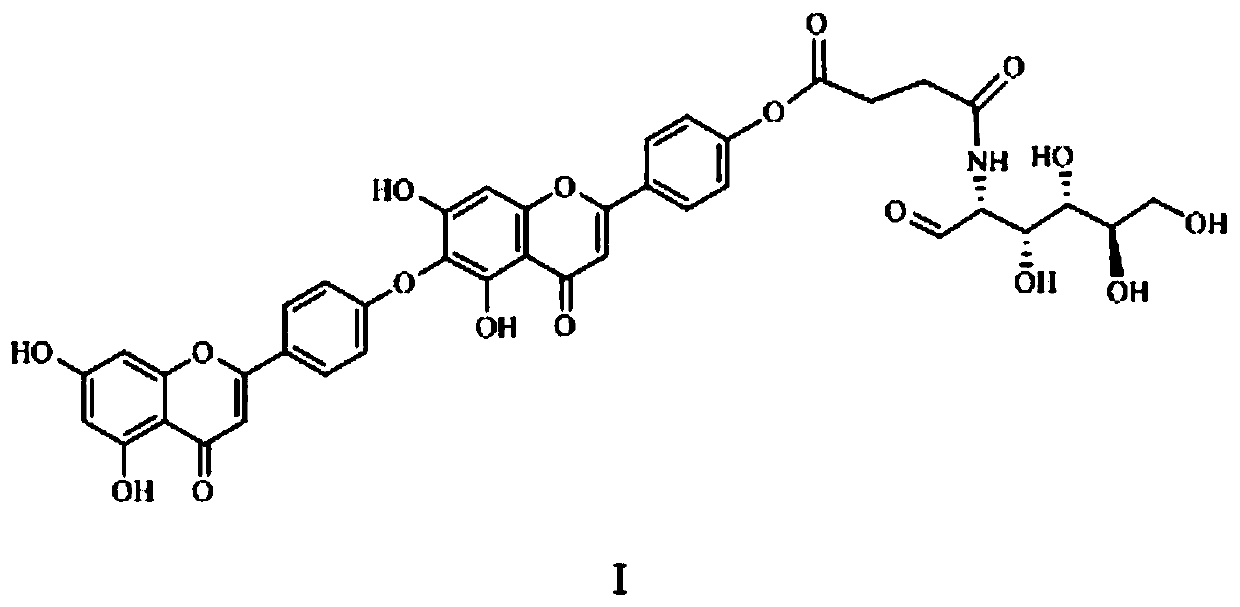
[0027] Cypress biflavone, yellow powder, easily soluble in DMSO. ESI-MSm / z: 537[M+H] - , mp 256~258℃, 1 HNMR (d-DMSO, 40...
Embodiment 2
[0028] Synthesis of Example 2 Cypress Biflavone Derivatives WG020
[0029] (1) Accurately weigh 10g of cypress biflavonoids, add 50ml of dry acetonitrile to dissolve, add 2ml of imidazole after complete dissolution, add 5.5g of TBSCl dropwise at room temperature, and react at room temperature for 1h after the dropwise addition. After the reaction was completed, add 100ml of dichloromethane and 100ml of water, separate layers, extract the aqueous layer with dichloromethane 3 times (50ml once), combine the extracts, wash with 100ml of water, wash with 100ml of saturated brine, and wash the organic layer with anhydrous sodium sulfate After drying and concentration, it was subjected to silica gel column chromatography (chloroform: acetone = 10:1) to obtain 7.8 g of yellow solid product tert-butyldiphenylcypress biflavone, which was dried for later use.
[0030] In addition to acetonitrile, the organic solvent used to dissolve cypress biflavone in the above-mentioned examples can a...
Embodiment 3
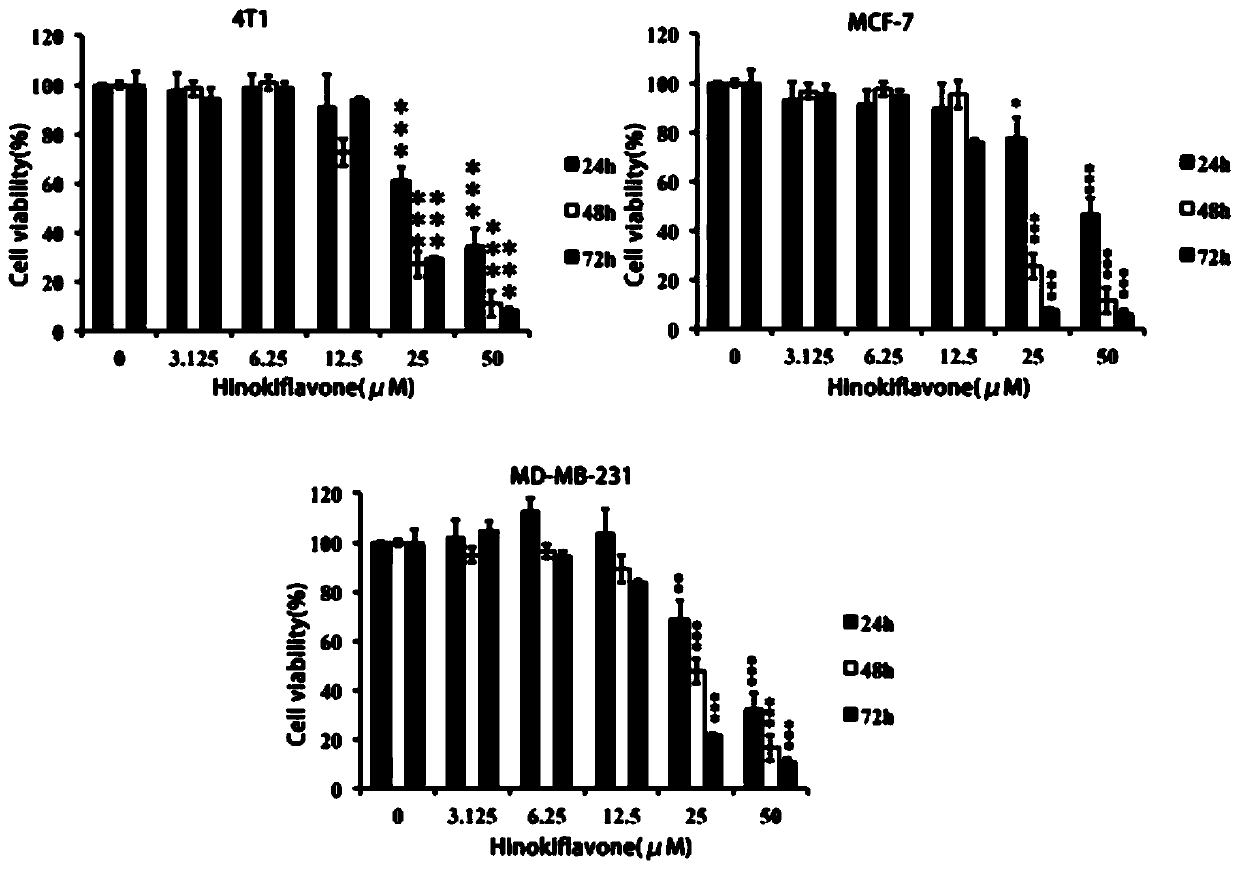
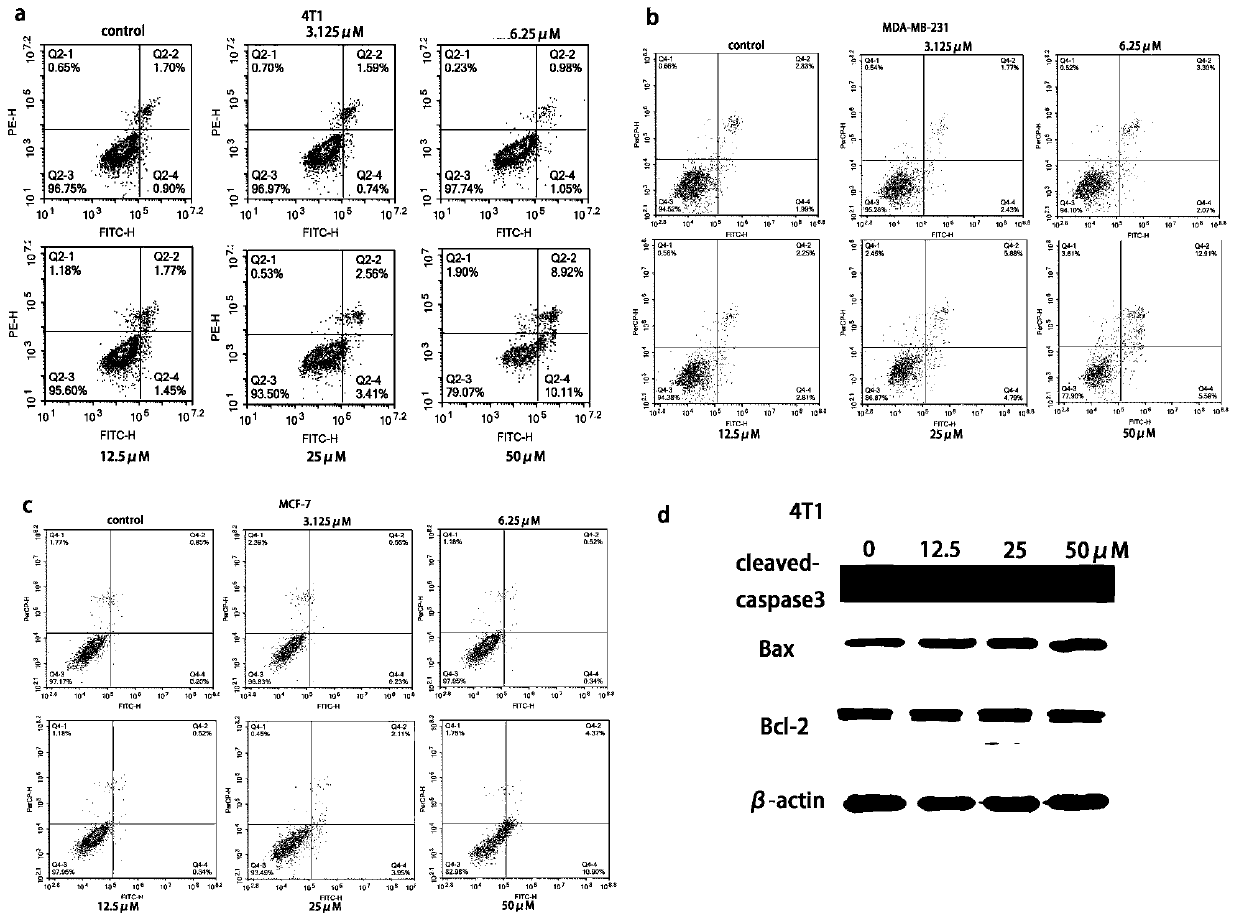
[0038] Example 3 Cypress biflavone derivative WG020 inhibits the proliferation of breast cancer cells
[0039] 1. Experimental materials
[0040] Test drug Cypress biflavone derivative WG020, light yellow powder, 98% purity, dissolved in DMSO, filtered and sterilized for storage, diluted with cell culture medium before use.
[0041] Reagents Human-derived breast cancer cells MDA-MB-231 and MCF-7, mouse-derived breast cancer cells 4T1, culture medium, trypsin, superior fetal bovine serum, tetramethyl azo blue powder (MTT powder ).
[0042] 2. Experimental equipment micro-adjustable pipette, cell counter, CO 2 Incubator, Millipore water purifier, low-temperature centrifuge, enzyme label detector, -80 ℃ ultra-low temperature refrigerator, constant temperature mixer, inverted microscope.
[0043] 3. Experimental process When the growth activity of the cells tends to be stable, spread the above three types of cells evenly in a 96-well plate, and add 100 μL of medium containing a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com