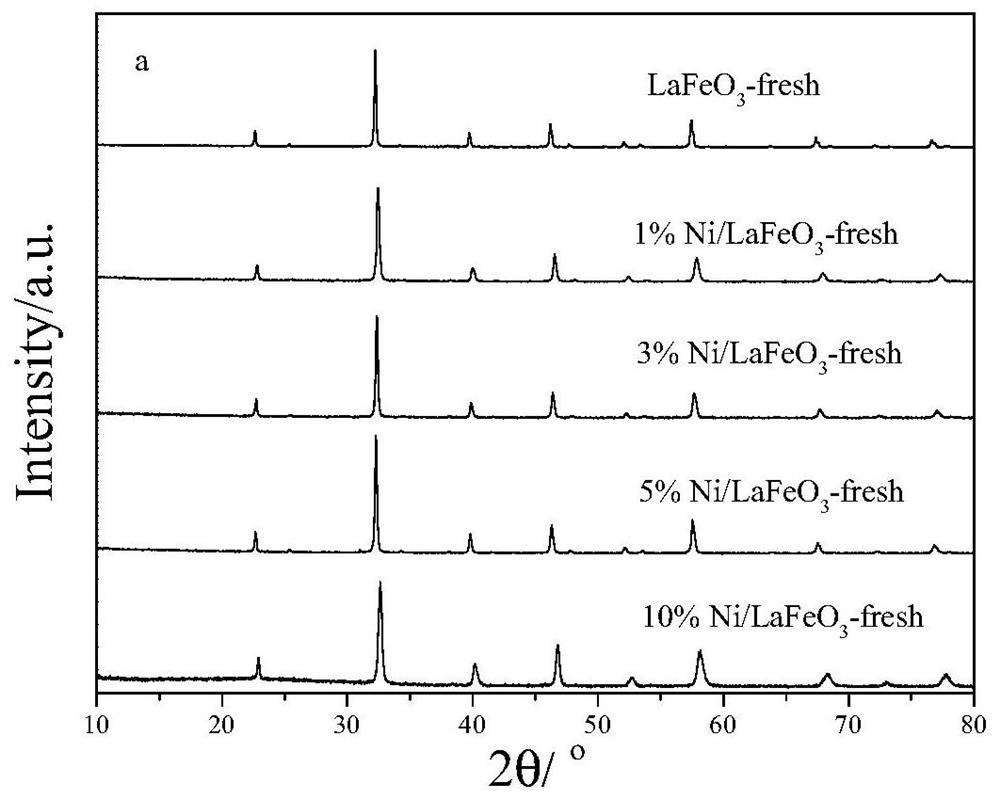
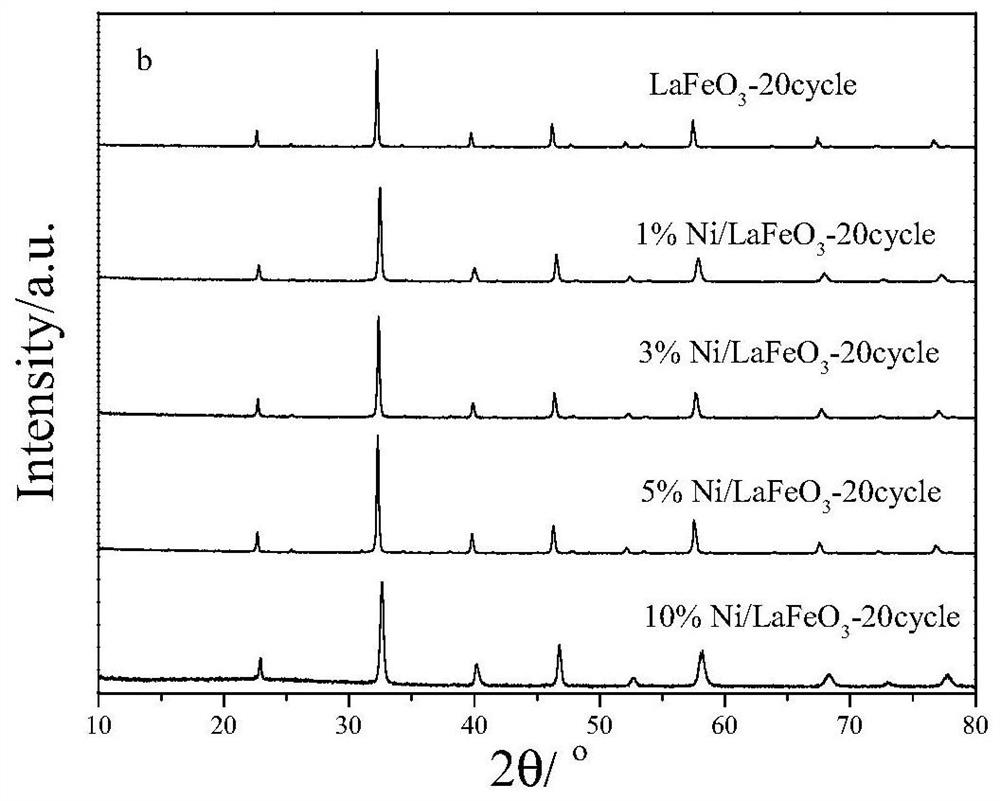
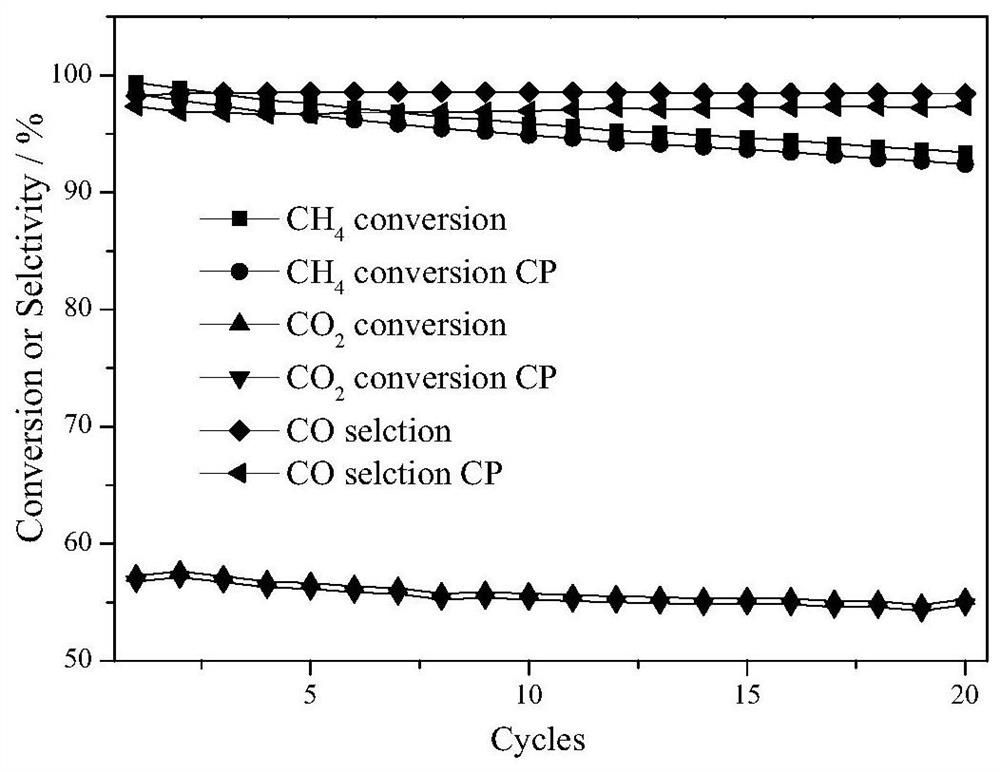
A kind of synthetic gas oxygen carrier produced by chemical chain partial oxidation of methane and its preparation method and application
An oxygen carrier and chemical chain technology, which is applied in the field of chemical chain partial oxidation of methane to synthesis gas oxygen carrier and its preparation, can solve the problems of reduced oxygen carrier performance, low methane conversion rate, poor cycle stability, etc., to maintain cycle stability , high reactivity, strong interaction effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] The preparation method of the above-mentioned chemical chain partial oxidation methane synthesis gas oxygen carrier comprises the following steps:
[0038] 1) Preparation of LaFeO 3 Carrier:
[0039] Prepared by sol-gel method: add lanthanum nitrate and iron nitrate precursors to the citric acid solution according to the molar ratio of 1:1, the amount of citric acid and the ratio of the total amount of matter between La and Fe cations are (2.0~ 3.0): 1. After completely dissolving, use ammonia water with a mass concentration of 5% to 28% to adjust the pH value to 5 to 8, then evaporate to dryness in a water bath at 60 to 100°C to a gel state, and dry at 60 to 180°C 2~24h, and finally roasted at 700~1400℃ for 1~12h to get LaFeO 3 carrier;
[0040] When the sol-gel method is used to prepare, preferably, the ratio of the amount of citric acid to the total amount of La and Fe cations is 2.5:1, and after being completely dissolved, ammonia water with a mass concentration ...
Embodiment 1
[0050] Dissolve lanthanum nitrate and ferric nitrate in deionized water at 60°C, the total concentration of La and Fe cations is 0.2mol / L, then quickly add ammonium carbonate solution with a concentration of 1mol / L to the solution, and adjust the pH to 8, Then stir rapidly at 60°C for 4h, stand and age for 2h, filter, wash, dry at 80°C for 12h, and then bake at 900°C for 4h to obtain LaFeO 3 carrier.
Embodiment 2
[0056] Dissolve lanthanum nitrate and ferric nitrate in deionized water at 60°C, the total concentration of La and Fe cations is 0.2mol / L, then quickly add ammonium carbonate solution with a concentration of 1mol / L to the solution, and adjust the pH to 8, Then stir rapidly (250-625r / min) at 60°C for 4h, let it stand for aging for 2h, filter, wash, dry at 80°C for 12h, and then roast at 900°C for 4h to obtain LaFeO 3 Carrier, weigh 2gLaFeO 3 Carrier, and then slowly pour it into the pre-prepared 0.344mol / L nickel nitrate solution, stir rapidly for 1 hour, dry at 60°C for 12h, and roast at 400°C for 4h to obtain the composite oxide oxygen carrier.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com