A value determining method for a sulfonamide veterinary drug residual matrix reference material in pork
A standard substance and matrix technology, which is applied in the field of analysis and detection, can solve the problems that the recovery rate and relative standard deviation need to be further optimized, affect the relative recovery rate and relative standard deviation of the drug, and cannot meet the value requirements of the matrix standard material. Avoid uneven quality, reduce content, and avoid the effect of adsorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0083] Preparation of blank pork: obtained from pigs fed without adding drugs from the farm;
[0084] Preparation of candidate matrix standard substances for sulfonamide veterinary drug residues in pork: Pigs raised without adding drugs were ordered from the farm, administered intravenously according to the pre-designed dosage, and positive samples were obtained.
Embodiment 1
[0085] The preparation of embodiment 1 standard working solution
[0086] Preparation of standard stock solution: Accurately weigh 10.0 mg (accurate to 0.1 mg) of sulfamethazine veterinary drug purity standard substance and sulfamethazine veterinary drug purity standard substance converted to 100% purity respectively, dissolve completely with methanol, transfer to 10 mL brown In a volumetric flask, dilute to the volume with methanol, shake well, and prepare a standard stock solution with a mass concentration of 1 mg / mL, and store it in a refrigerator at -20°C in the dark, with a validity period of 12 months; accurately weigh the isotope-labeled solution whose purity is converted to 100%. Sulfamethazine and isotope-labeled sulfadiazine each 1.0mg (accurate to 0.1mg), completely dissolved in methanol, transferred to a 10mL brown volumetric flask, distilled to volume with methanol, shaken well, and prepared to a mass concentration of 0.1mg / mL Isotope-labeled standard stock soluti...
Embodiment 2
[0089] Embodiment 2 matrix standard substance pretreatment
[0090] (1) Sample preparation: take the carcass muscle on both sides of the pig, cut into meat slices of appropriate size, grind 3 times with a meat grinder with an aperture of 5 mm, and manually mix well;
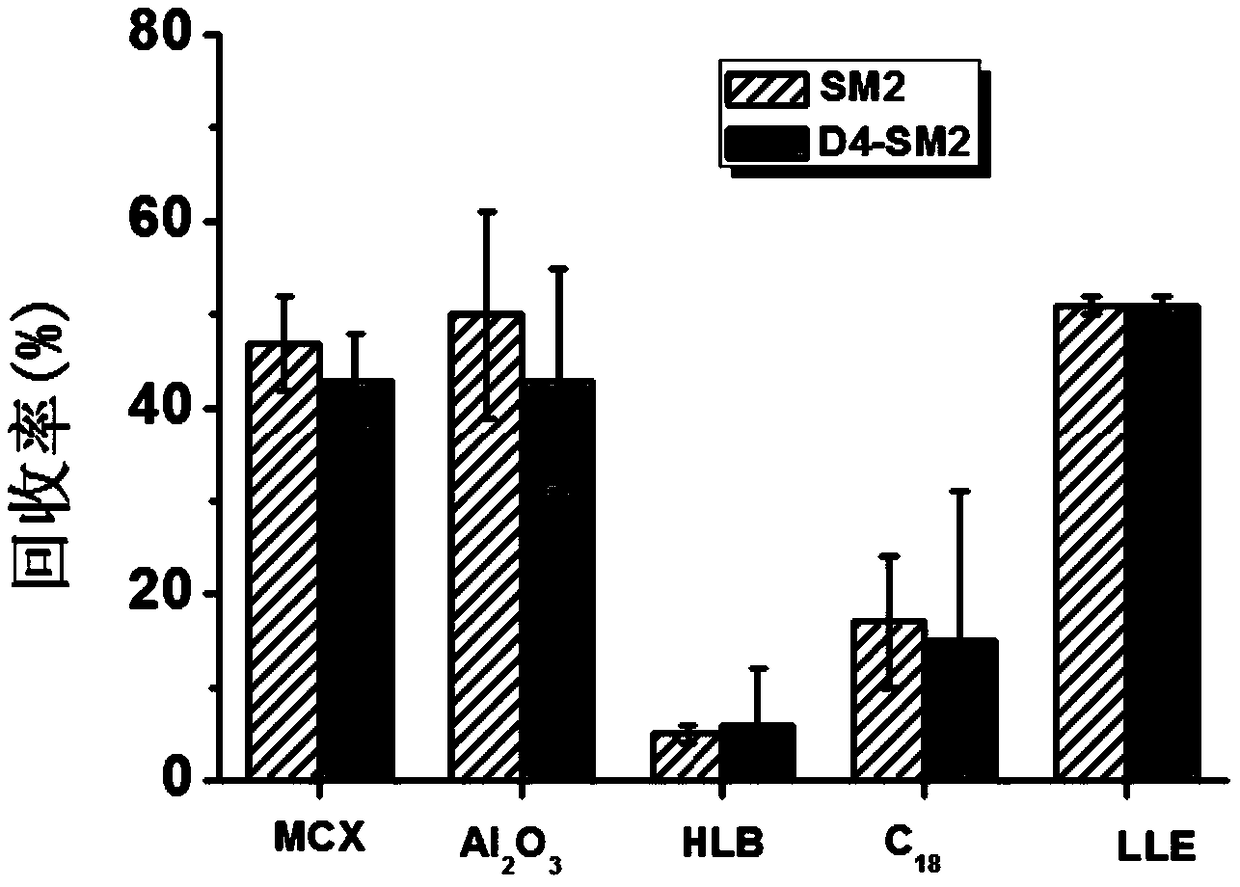
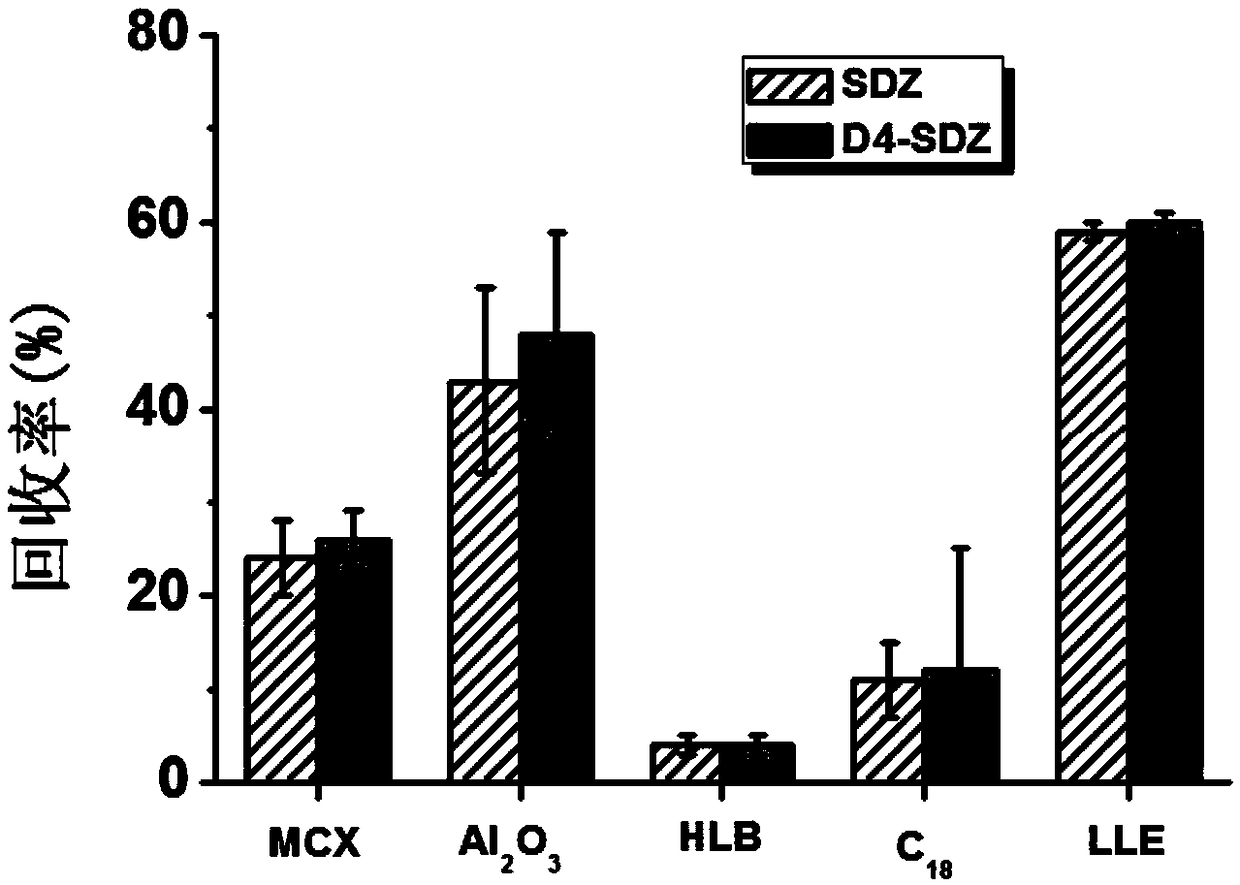
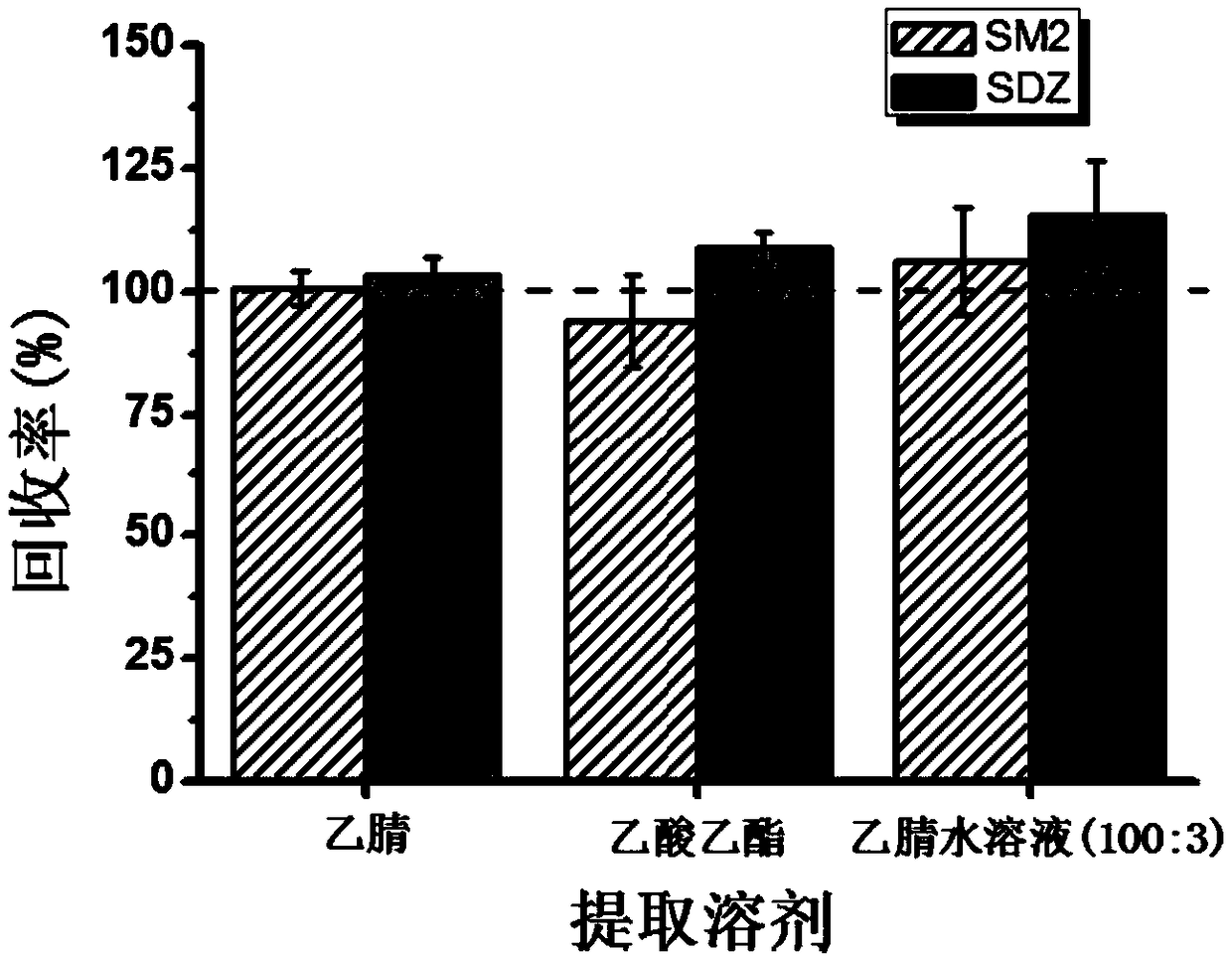
[0091](2) Extraction: Accurately weigh 3.00g (accurate to 0.01g) of pork and place it in a 50mL centrifuge tube, add 300μL isotope labeled standard mixed solution with a concentration of 1μg / mL, mix on a vortex mixer for 1min, add 20mL Acetonitrile, homogenate for 2 minutes, centrifuge at 9000r / min (4°C) for 2 minutes, pass the obtained supernatant through a 70g anhydrous sodium sulfate column (infiltrated with acetonitrile in advance) to remove the water, and transfer it to a 100mL chicken heart bottle, repeat the operation Three times, the obtained extract was dried by rotary evaporation in a 50°C water bath, and 3 mL of n-hexane-saturated mobile phase (acetonitrile-0.1% formic acid aqueous solution (5:95)) was...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com