Tetranectin mimetic peptide tnp and its application
A technology that mimics peptides and linkins, applied in the field of biomedicine, can solve the problems that the role has not been reported, and the function and mechanism of TN are not clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
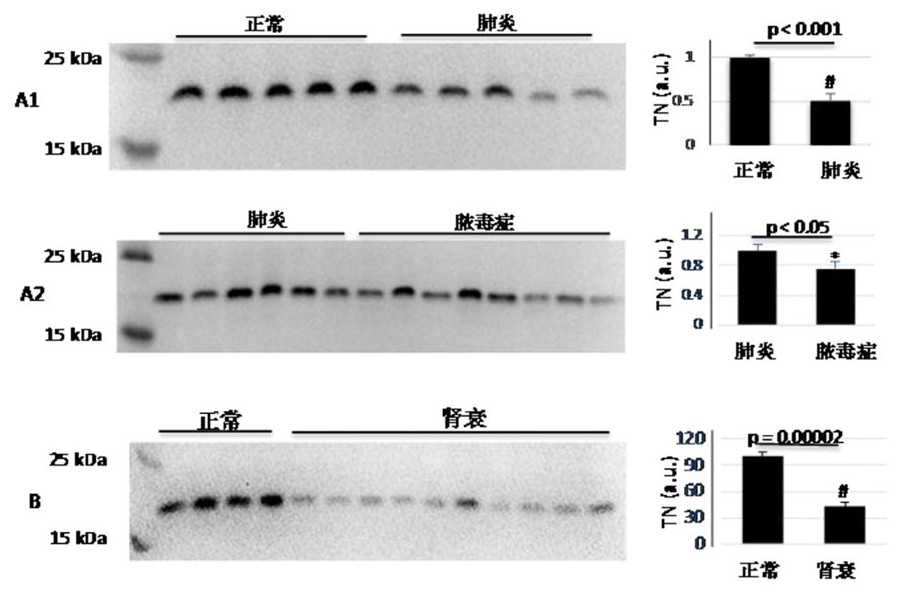
[0032] Example 1 Correlation between tetranectin TN and the pathological process of sepsis or acute kidney injury
[0033] Normal subjects (10 cases), pneumonia patients (27 cases), sepsis patients (37 cases), and renal failure patients (17 cases) were selected for the experiment, and western blotting (western blotting, Li W et al. (2018) ) Connexin 43hemichannel as a novel mediator of sterile and infectious inflammatory diseases. Scientific Reports 8(1): 166) Detect the level of TN in the plasma, separate 0.25μL plasma protein, and the plasma protein in the polyacrylamide gel with a mass concentration of 12% After electrophoresis separation and transfer to cellulose acetate membrane, the results of the TN protein band displayed by anti-TN antibody are as follows: figure 1 as shown, figure 1 The middle left bar chart (i.e. figure 1 The shades of the band colors in A1, A2, and B) reflect the difference in the amount of protein, and the gray value represents the relative amoun...
Embodiment 2
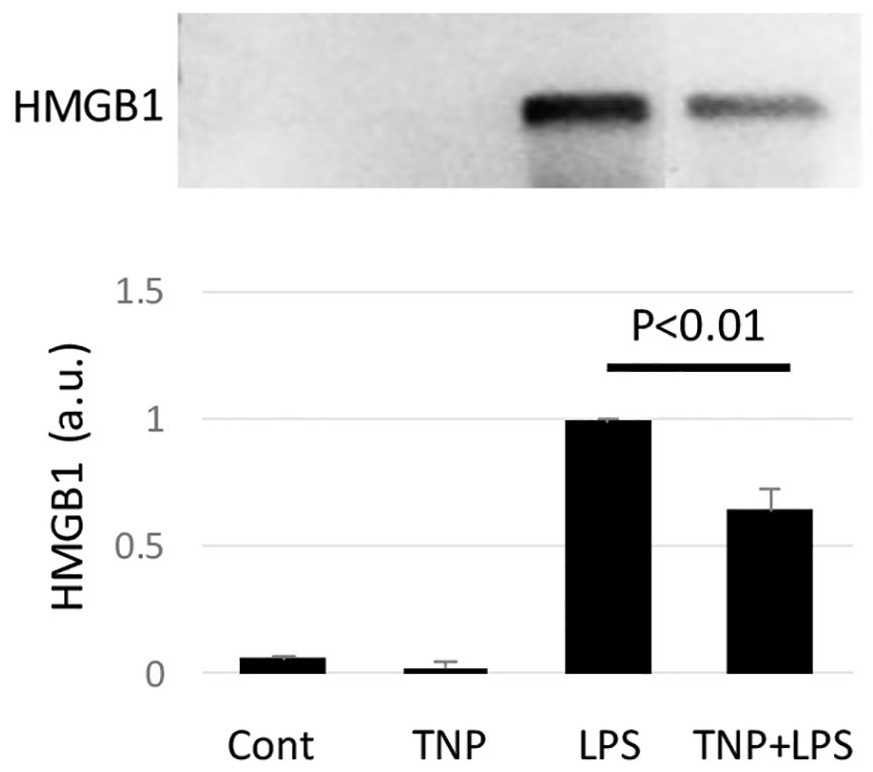
[0035] Example 2 Tetranectin mimetic peptide TNP inhibits the release of inflammatory factor HMGB1 from vascular endothelial cells
[0036] The specific composition of the culture medium for human umbilical cord vascular endothelial cells is Dulbecco's Modified Eagle's Medium, namely DMEM, which contains 20% fetal bovine serum, 100 units / ml of penicillin and 100 micrograms / ml of streptomycin. After washing with DMEM without serum and antibiotics, the cells were divided into control group (Cont, without adding any components), TNP group (containing 20 μg / ml TNP), LPS group (containing 1 μg / ml bacterial endotoxin LPS ) and the TNP+LPS group (containing 20 μg / ml of TNP and 1 μg / ml of LPS. After 20 hours, the culture media were collected and concentrated, and then immunoblotting was used to show HMGB1 in them, as figure 2 As shown (sample number n=4, p figure 2 Down).
[0037] Depend on figure 2 It can be seen that the cell culture medium without TNP or LPS (i.e. Cont) contain...
Embodiment 3
[0038] Example 3 Acetylation of tetranectin mimetic peptide TNP can effectively prevent TNP hydrolysis
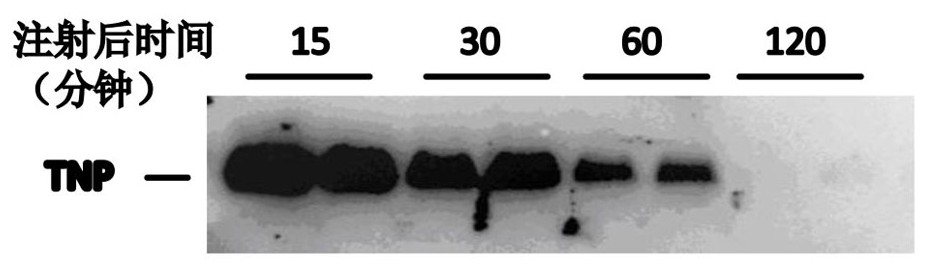
[0039] In this experiment, 8 Balb / c male mice (body weight about 25 g) at 8 weeks after birth were used. After the mice were intraperitoneally injected with TNP (8 mg / kg body weight), the mice were sacrificed at 15 minutes, 30 minutes, 1 hour and 2 hours respectively, and the blood was collected, and the plasma was extracted by centrifugation and separated by electrophoresis. Then TNP in plasma was detected by the combination of horseradish peroxidase-coupled streptavidin (streptavidin-HRP) and biotin (biotin) in TNP, the results were as follows image 3 shown.
[0040] Depend on image 3 It can be seen that 15 minutes after the injection of TNP, the TNP level in the mouse plasma is the highest, indicating that after intraperitoneal injection, TNP can be quickly absorbed into the circulation system, and then gradually decreased over time, but it can still be detected 2 ho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com