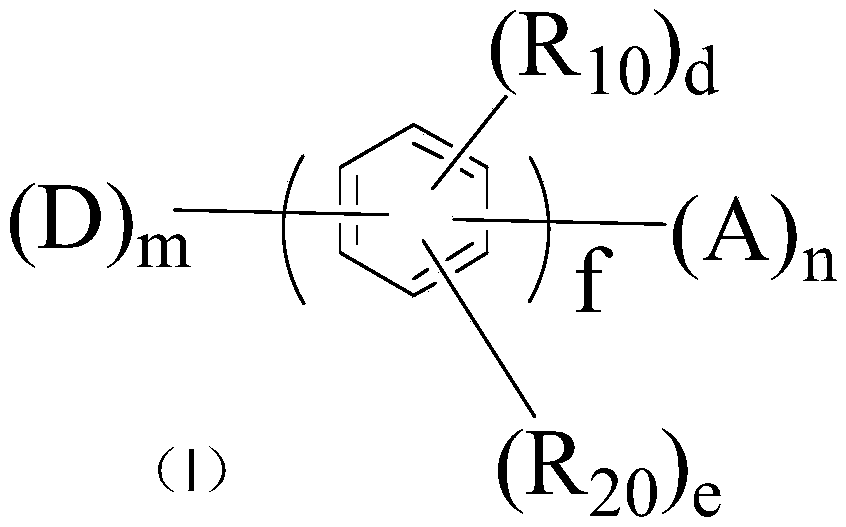Aromatic compound and organic light-emitting display device
A technology of aromatic compounds and heteroaryl groups, applied in the field of organic electroluminescent materials, to achieve the effect of improving solution processability, improving solubility, and small FWHM
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Synthesis of intermediate S2
[0105]
[0106] At room temperature, add 20ml of concentrated sulfuric acid to a 50ml single-necked flask, then add 6mL of bromobenzene S1 (57mmol), stir at room temperature for half an hour to obtain a white turbid liquid, then add 1.0g of mercaptosalicylic acid (6.5 mmol). Stir at room temperature for 24 hours, then heat at 100°C for 2-3 hours, after cooling to room temperature, carefully pour into ice water, filter with suction to obtain a solid, then add 20% NaOH aqueous solution, stir for 2 hours, filter with suction, wash with water until neutral, and get Yellow solid S2 (5.2 mmol, 80%).
[0107] 1 H NMR (400MHz, CDCl 3 , ppm): 7.70-7.90 (s, 2H), 7.40-7.60 (m, 4H), 7.30 (m, 1H). MALDI-TOF MS: m / z calcd for C 13 h 7 BrOS: 289.9; found: 290.0
[0108] Synthesis of intermediate S3
[0109]
[0110] At room temperature, add 40mL glacial acetic acid and 20mL dichloromethane to a 50mL single-necked flask, add raw material int...
Embodiment 2
[0124]
[0125] Under the condition of nitrogen protection, weigh S8 (20mmol), add 60mL of acetic acid, and add 24mmol of liquid bromine dropwise under the condition of stirring, and stir the resulting mixed solution at 80°C for 5h. Use NaHSO 3 The excess bromine element was quenched with aqueous solution, extracted with dichloromethane (100mL×3), the organic phase was collected, and washed with anhydrous Na 2 SO 4 Dry processing. After filtration, the solvent was distilled off under reduced pressure with a rotary evaporator to obtain a crude product. The crude product was purified by gradient elution of silica gel column chromatography, and finally purified by recrystallization from n-hexane to obtain solid powder S9 (16.8 mmol, 84%).
[0126] MALDI-TOF MS: m / z calcd for C 12 h 7 BrS 2 :293.9; found: 293.8
[0127]
[0128] At room temperature, add 40mL glacial acetic acid and 20mL dichloromethane into a 50mL single-necked flask, add raw material intermediate S9 ...
Embodiment 3
[0142]
[0143] S13 (8mmol), S7 (10.5mmol), (dibenzylideneacetone) dipalladium (0) (0.05mmol), sodium tert-butoxide (14mmol), 4,5-bisdiphenylphosphine-9,9 - Dimethylxanthene (0.2 mmol) was put into a 50 mL three-necked flask, and while stirring, degassing and nitrogen replacement were repeated three times rapidly, and 20 mL of toluene was added through a syringe. The mixture was heated to reflux for 3 hours under nitrogen flow. After the reaction, water was added to the reaction solution left to cool to room temperature, extracted with dichloromethane, and washed with saturated brine. After drying the organic layer with anhydrous sodium sulfate, the solvent was distilled off and purified by column chromatography to obtain intermediate S14 (5.3 mmol, 66%).
[0144] MALDI-TOF MS: m / z calcd for C 33 h 38 BrN: 527.2; found: 527.5
[0145]
[0146] In a 250ml three-necked flask, first mix S14 (30mmol), pinacol diborate (36mmol), (1,1'-bis(diphenylphosphino)ferrocene)dichl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com