Industrialized preparation method of tantalum-aluminum-codoped lithium-lanthanum zirconate solid-state electrolyte
A solid-state electrolyte, lithium lanthanum zirconate technology, used in chemical instruments and methods, tantalum compounds, inorganic chemistry, etc., can solve the problems of high experimental repeatability, high ionic conductivity, high product density, and easy generation of miscellaneous phase conductivity. , to achieve the effect of simple preparation process, improved electrical conductivity and high density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
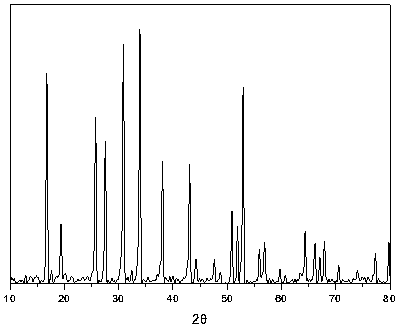
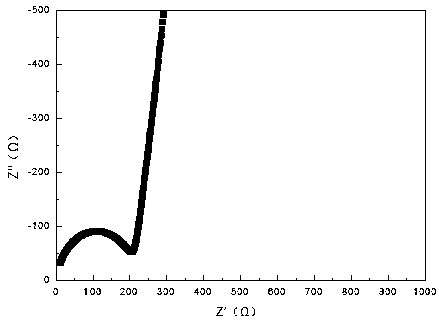
Image
Examples
Embodiment 1
[0025] 14.49g (10% excess of Li source) lithium hydroxide monohydrate powder, 24.45g lanthanum oxide powder (the mass after sintering at 900 ℃ for 12h), 10.78g zirconium dioxide, 2.7625g (X=0.25) five Tantalum oxide and 50 ml of isopropanol were added, and then stirred evenly to ensure adequate mixing, and zirconia balls with a ball-to-material ratio of 5:1 were added and placed in a ball mill for 12 hours at 500 rpm. After the ball mill is finished, take it out and filter it, put it in an oven for 6 hours at 80°C, take it out and grind it with a mortar for 30 minutes after it is completely dried, and then sieve it after grinding it into powder. After collecting and sieving, the powder was placed in a magnesia crucible for calcination at 850°C for 12 hours. After cooling, it was taken out and ground again for 30 minutes to form a powder. Part of the powder was sieved and collected as master powder. Repeat the ball milling work for another part of the powder, weigh 0.51g of alu...
Embodiment 2
[0027]13.90g (10% excess of Li source) lithium hydroxide monohydrate powder, 24.45g lanthanum oxide powder (the mass after sintering at 900°C for 12h), 32.20g zirconium nitrate, 5.525g (X=0.5) pentoxide Add tantalum and 50ml of absolute ethanol, stir evenly to ensure full mixing, add zirconium dioxide balls with a ball-to-material ratio of 5:1 and place them in a ball mill for 12h at 500rpm. After the ball mill is finished, take it out and filter it, put it in an oven for 6 hours at 80°C, take it out and grind it with a mortar for 30 minutes after it is completely dried, and then sieve it after grinding it into powder. After collecting and sieving, the powder was placed in a magnesia crucible for calcination at 900°C for 9 hours. After cooling, it was taken out and ground again for 30 minutes to form a powder. Part of the powder was sieved and collected as master powder. Repeat the ball milling work for another part of the powder, weigh 0.51g of alumina into the obtained powde...
Embodiment 3
[0029] 19.15g (20% excess of Li source) lithium oxalate powder, 28.49g lanthanum hydroxide powder (the mass after sintering at 900℃ for 12h), 14.33g zirconium hydroxide, 2.21g (X=0.2) dioxide pentoxide Tantalum and 50 ml of isopropanol were added, and then stirred evenly to ensure adequate mixing, and zirconia balls with a ball-to-material ratio of 5:1 were added and placed in a ball mill for 12 hours at 500 rpm. After the ball mill is finished, take it out and filter it, put it in an oven for 6 hours at 80°C, take it out and grind it with a mortar for 30 minutes after it is completely dried, and then sieve it after grinding it into powder. After collecting and sieving, the powder was placed in a magnesia crucible for calcination at 800°C for 12 hours. After cooling, it was taken out and ground again for 30 minutes to form a powder. Part of the powder was sieved and collected as master powder. Repeat the ball milling work for another part of the powder, weigh 0.51g of alumina ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com