Separation preparation method of mezlocillin sodium impurity A
A technology of refining method of mezlocillin sodium, which is applied in the separation and preparation of impurity A of mezlocillin sodium and the refining of impurities of antibiotic drug compounds, which can solve the difficulties in the synthesis of impurity A, low product purity, instability and easy degradation, etc. problems, to achieve the effect of shortening the separation time, simple process and stable product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
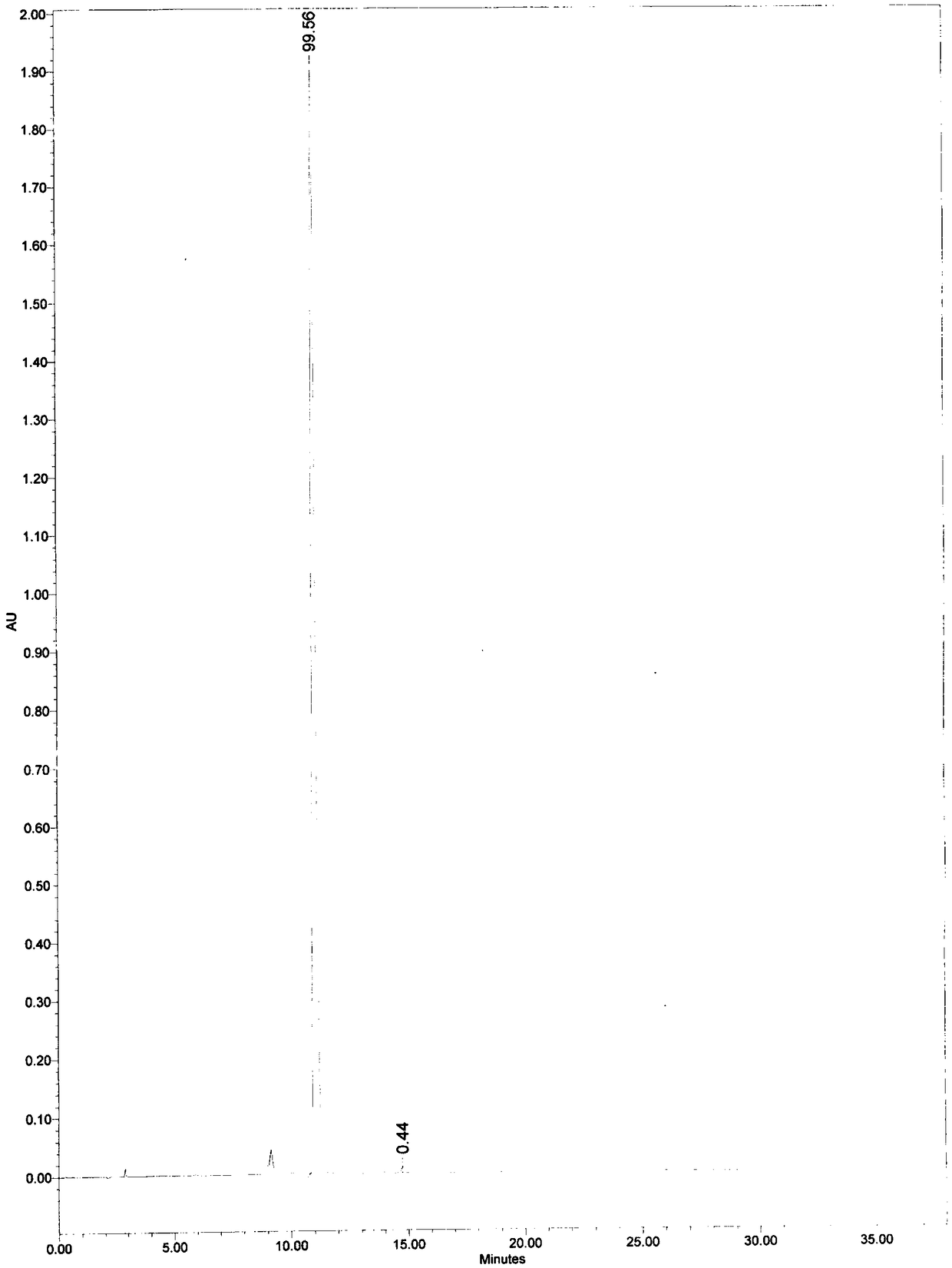
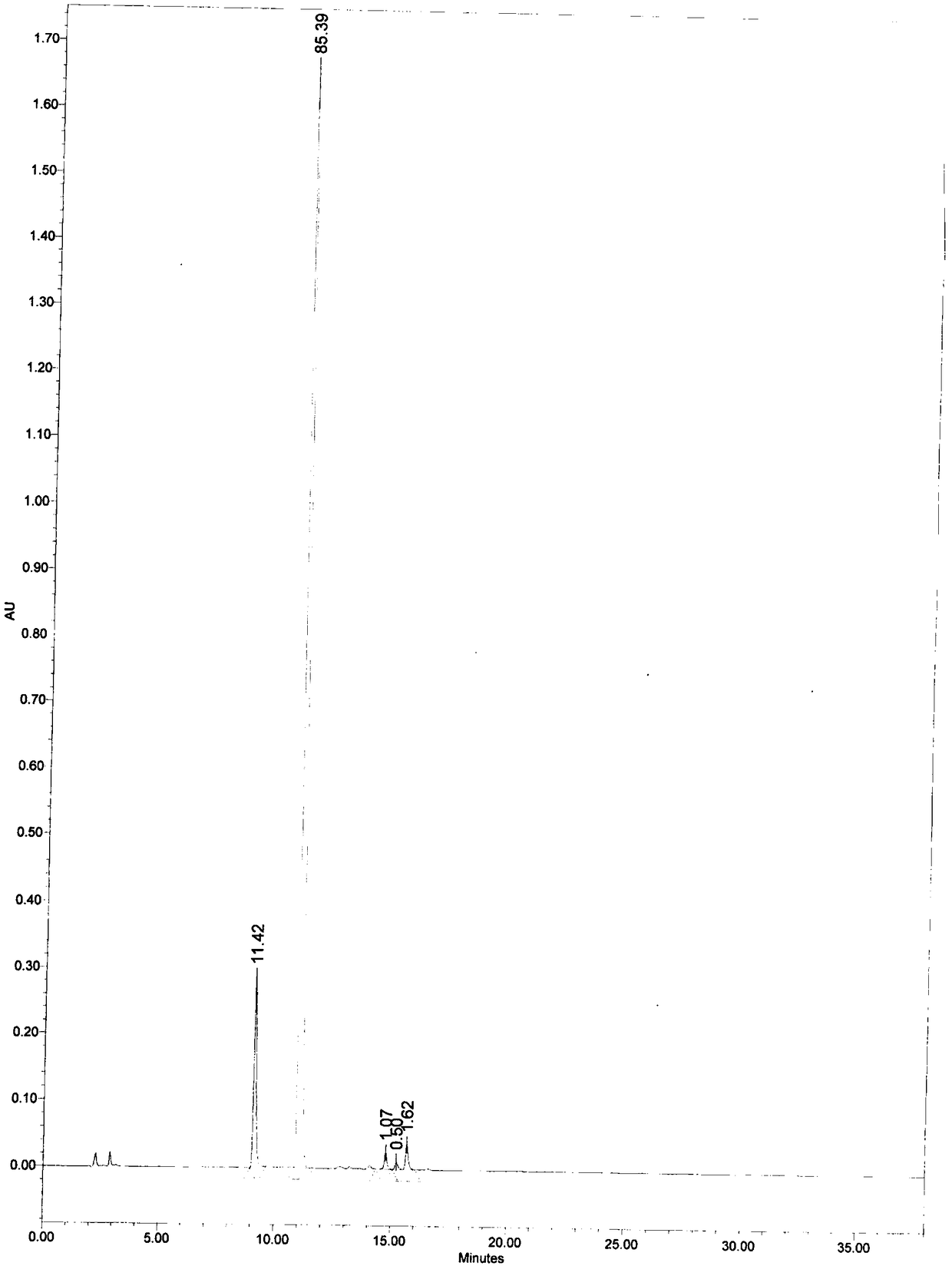
[0049] At room temperature, add 60 mL of 0.1 mol / mL potassium hydroxide solution to 2.0 g of mezlocillin sodium, stir for 30 minutes, then add 0.5 mol / mL hydrochloric acid solution dropwise to adjust the pH value of the solution to 3, add 20 g of calcium chloride solid was stirred for 5 minutes, filtered, rinsed once with 5 mL of water, then rinsed once with 5 mL of ethanol, and dried at room temperature to obtain 1.1 g of solid. According to the 2015 edition of the Chinese Pharmacopoeia, octadecylsilane bonded silica gel is used as a filler; the mobile phase is a buffer solution (take 4.9 g of potassium dihydrogen phosphate and 0.45 g of dipotassium hydrogen phosphate, dissolve and dilute to 1000 mL with water): acetonitrile, Isocratic elution at a volume ratio of 80:20, flow rate 1.0mL / min; column temperature 30°C, detection wavelength 210nm; retention time 11.1min. The calculated yield was 70%.
Embodiment 2
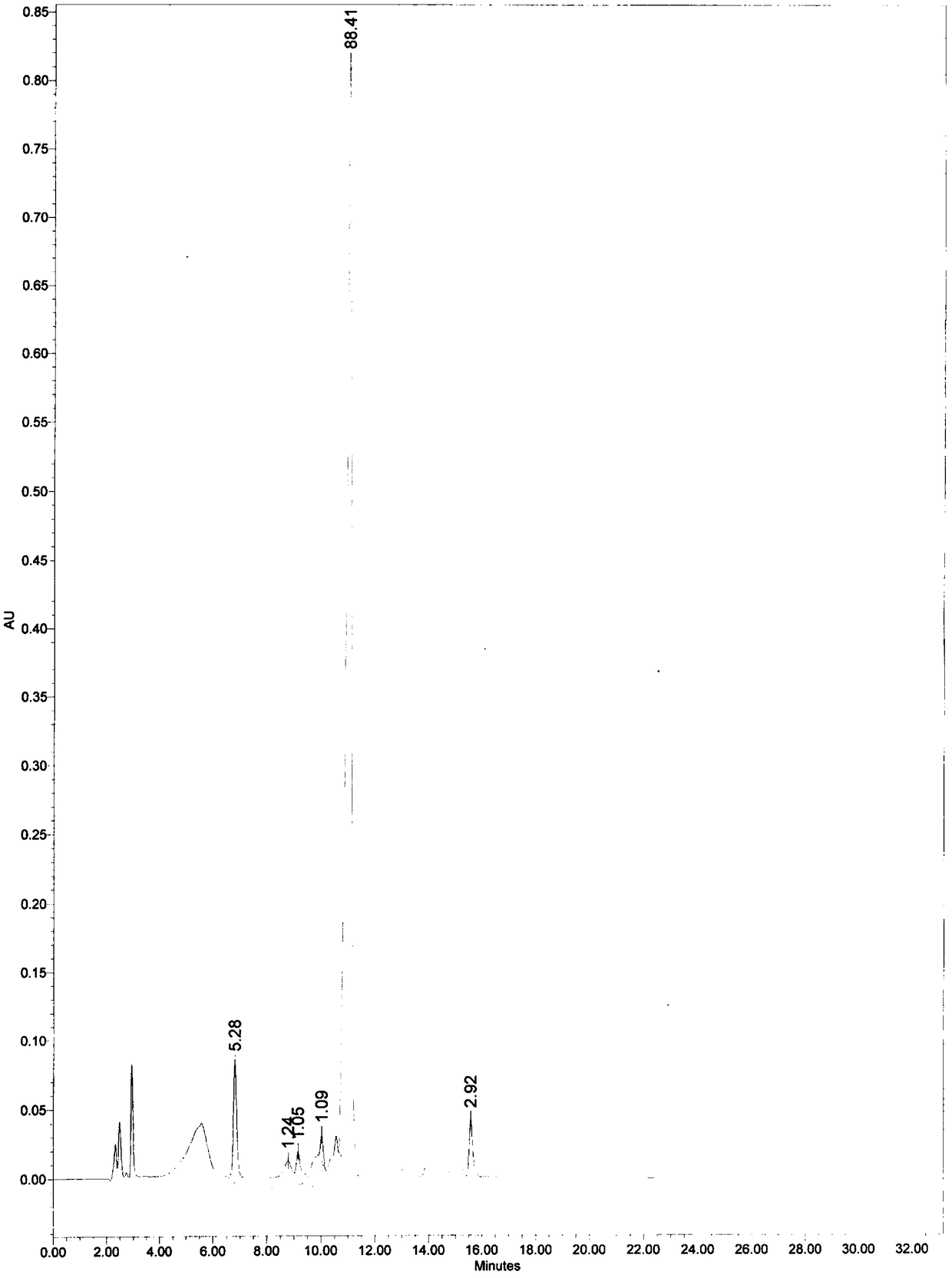
[0051] At room temperature, add 60 mL of 0.1 mol / mL sodium hydroxide solution to 2.0 g of mezlocillin sodium, stir for 30 min, add phosphoric acid solution drop by drop, adjust the pH value of the solution to 4, add 20 g of calcium chloride solid After stirring for 5 min, filter, rinse once with 5 mL of water, then rinse once with 5 mL of ethanol, and dry at room temperature to obtain 1.1 g of solid. The calculated yield was 67%.
Embodiment 3
[0053] At room temperature, add 60 mL of ammonia water to 2.0 g of mezlocillin sodium, stir for 30 min, then add acetic acid solution drop by drop, adjust the pH value of the solution to 5, then add 15 g of potassium chloride solid and stir for 10 min, filter, and use Rinse twice with 5 mL of acetone and dry at room temperature to obtain 1.2 g of solid with a yield of 52%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com