A method for producing extracellular ferulic acid esterase from Escherichia coli
A technology of ferulic acid esterase and Escherichia coli, applied in the direction of biochemical equipment and methods, using vectors to introduce foreign genetic material, enzymes, etc., to achieve the effect of simplifying purification and utilization, and promoting industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: Cloning of Lactobacillus amylophagous ferulic acid esterase gene
[0058] Take 1-3ml of Lactobacillus amylophagicus cultured overnight and centrifuge at 6000rpm for 3min to collect the bacteria. Resuspend in 567μl TE buffer (10mM Tris-HCl, 1mM EDTA, pH 8.0), add 25μL 50mg / ml lysozyme, mix well, and treat in 37℃ water bath for 1h. Add 30 μL of 10% (mass percentage) SDS, 3 μL of 20 mg / ml proteinase K, and treat in a water bath at 37° C. for 1 h. Add 100 μL of 5M NaCl and 80 μL of CTAB / NaCl solution (10% (mass percentage) CTAB, 0.7 mol / L NaCl) and treat in a water bath at 65° C. for 10 minutes. Add an equal volume of chloroform isoamyl alcohol (volume ratio 24:1) for extraction, centrifuge at 12,000 rpm for 5 min, and retain the supernatant. Add RNase A solution with a final concentration of 100 μg / mL, and treat in a water bath at 37°C for 30 minutes. Then use an equal volume of phenol-chloroform-isoamyl alcohol (volume ratio 25:24:1) and chloroform-isoamy...
Embodiment 2
[0060] Embodiment 2: Construction of ferulic acid esterase gene recombinant Escherichia coli expression strain
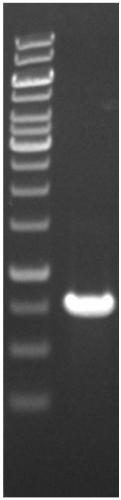
[0061] Plasmid pET-22b was extracted and digested with NdeI and XhoI, and the digested products were recovered by gel. Mix the digested target gene with the vector pET-22b at a molar ratio of 1:5, and use T 4 DNA ligase was ligated overnight at 16°C, and then the ligated products were added to E. coli DH5α competent cells for heat shock transformation. The recovered cultured bacterial solution was concentrated and spread on LB (Amp resistant) plates, and cultivated overnight at 37. Pick the transformant and check it by enzyme digestion. After extracting the plasmids from the successfully verified DH5α transformants, they were transformed into E.coli BL21(DE3), and the BL21(DE3) transformants were screened out on LB (Amp resistant) plates.
Embodiment 3
[0062] Embodiment 3: Utilize IPTG to induce Escherichia coli genetic engineering strain to secrete and express ferulic acid esterase
[0063] Pick BL21(DE3) transformants into 5 mL LB containing Amp antibiotics, transfer to 100 mL LB medium at 2% after overnight culture, and culture at 37°C until the OD value is about 0.6, add IPTG with a final concentration of 0.5 mM, Continue to culture for 24h to induce protein expression.
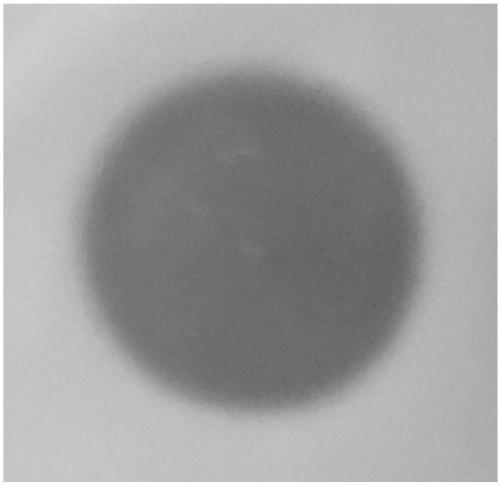
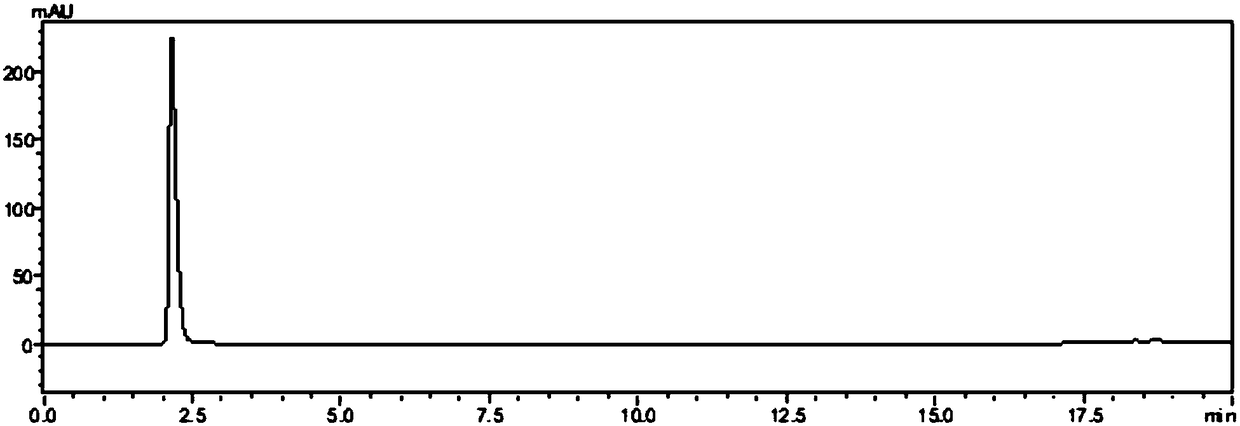
[0064] Preliminary test of ferulic acid esterase activity secreted by Escherichia coli genetically engineered strains: the induced expression of Escherichia coli culture fluid was centrifuged at 13000rpm for 10min, and the collected supernatant was filtered with a 0.22μm filter membrane to be its extracellular secretion fraction. Take 200uL of the secretory fraction and add it to the Oxford cup placed on the ferulic acid esterase activity detection medium. In 10% ethyl ferulic acid solution of N,N-dimethylformamide, after standing at 37°C for 6 hours, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com