Low-cost iron phosphate ammonia-nitrogen-containing wastewater treatment method
A technology for ammonia nitrogen wastewater and a treatment method, applied in the field of industrial wastewater treatment, can solve the problems of low economic value and high nitrogen recovery process cost, and achieve the effects of excellent economic value, reduced evaporation cost and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation process of iron phosphate is as follows:
[0041] Ferric phosphate reaction stock solution (NH 4 h 2 PO 4 , FeSO 4 、H 3 PO 4 mixture) and H 2 o 2 Mixing, oxidation-reduction reaction occurs, the reaction formula is as follows:
[0042] 2NH 4h 2 PO 4 +2FeSO 4 +H 2 o 2 =2FePO 4 ↓+2NH 4 HSO 4 +2H 2 o
[0043] H in the reaction material 3 PO 4 Does not directly participate in the reaction, its main function is to adjust the acidity in the reaction process, PO in the ferric phosphate mother liquor 4 3- less.
[0044] The reaction slurry is hydraulically filtered to obtain iron phosphate filter cake and iron phosphate mother liquor, and the iron phosphate filter cake is washed and calcined to obtain iron phosphate products; the pH value of the iron phosphate mother liquor is 1-2.5, mainly containing NH 4 HSO 4 、H 3 PO 4 , that is, the ferric phosphate mother liquor mainly contains NH 4 + 、H + , SO 4 2- 、PO 4 3- , In addition, i...
Embodiment 1
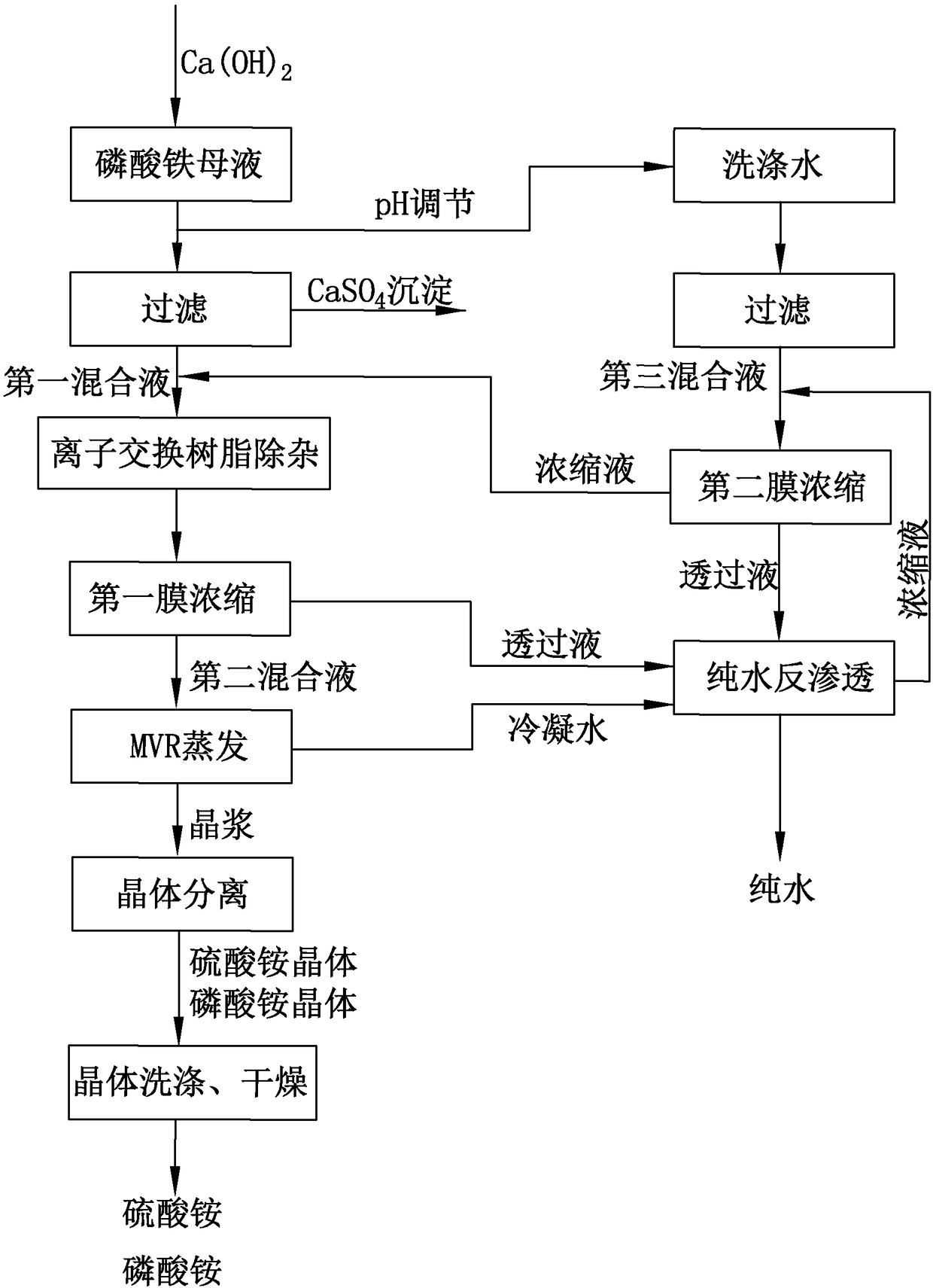
[0049] see figure 1 , is a schematic flow chart of the low-cost ferric phosphate ammonia nitrogen-containing wastewater treatment method provided by the present invention. A method for treating waste water containing ammonia nitrogen with low-cost ferric phosphate, comprising the steps of:
[0050] Step S1, adding an appropriate amount of Ca(OH) to the ferric phosphate mother liquor 2 , generate calcium sulfate precipitate, filter the precipitate to obtain the first mixed solution, the first mixed solution mainly contains NH 4 + , SO 4 2- and PO 4 3- ;
[0051] The main reaction equation is as follows:
[0052] Ca(OH) 2 +2NH 4 HSO 4 =CaSO 4 ↓+(NH 4 ) 2 SO 4 +2H 2 O (1)
[0053] Impurity removal reaction:
[0054] Fe 3+ +3OH - =Fe(OH) 3 ↓ (2)
[0055] In this reaction, the pH value of the neutralization reaction system is controlled to be 6-8 to avoid PO in the system 4 3- with Ca 2+ The reaction produces a calcium phosphate precipitate.
[0056] Thus,...
Embodiment 2
[0081] A method for treating waste water containing ammonia nitrogen with low-cost ferric phosphate, comprising the steps of:
[0082] Step S1, adding an appropriate amount of Ca(OH) to the ferric phosphate mother liquor 2 , generate calcium sulfate precipitate, filter the precipitate to obtain the first mixed solution, the first mixed solution mainly contains NH 4 + , SO 4 2- and PO 4 3- ;
[0083] In this process, Ca(OH) 2 The purity is 95%, and it is mixed with the SO in the iron phosphate mother liquor 4 2- The molar ratio is 1:2; the mass concentration of the first mixed solution obtained by filtration is 4%, and the pH value is 6.
[0084] The filtered first mixed solution is subjected to ion exchange resin removal of impurities in the resin adsorption tower to further remove iron ions in the first mixed solution, so that the content of Fe ions in the first mixed solution is lower than 3-5ppm.
[0085] Step S2, performing first membrane concentration on the fir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com