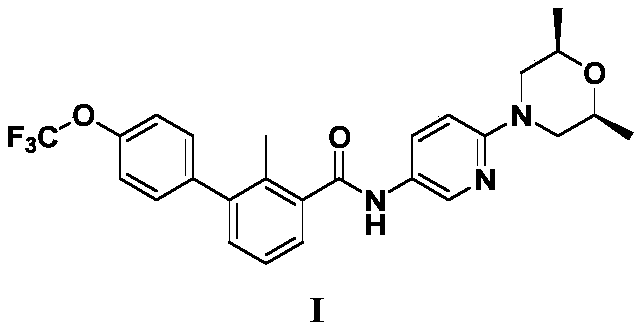
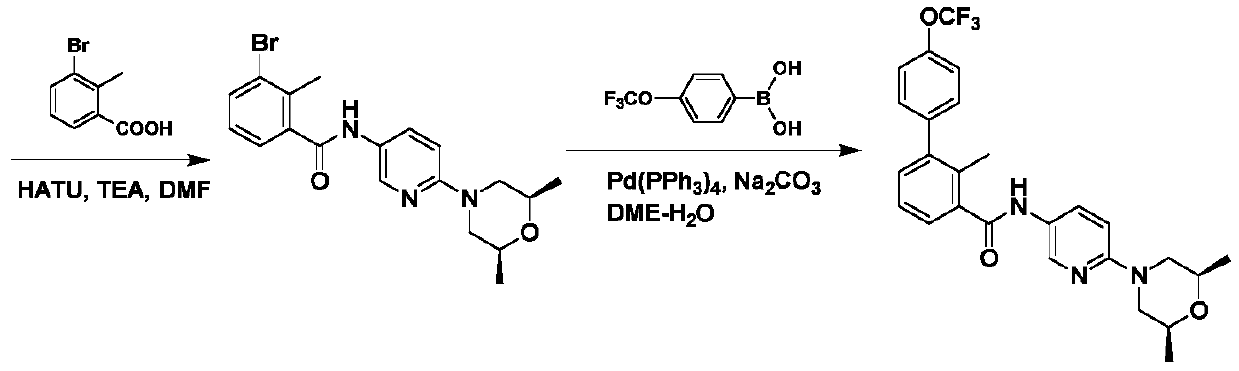
A kind of Sony Gibb intermediate and the preparation method of Sony Gibb
A technology of intermediates and solvents, applied in the field of medicinal chemistry, can solve the problems of unreasonable process route design, high cost and high price of Sony Gibb
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
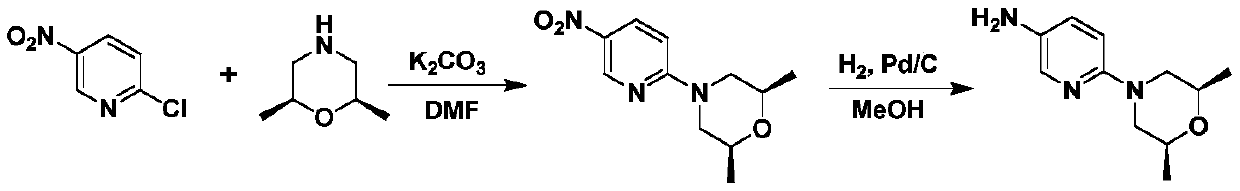
[0074] Example 1: Preparation of N,N-bis(2S-2-hydroxypropylamino)-5-nitropyridine (Ⅲ1)
[0075] In the 500 milliliter stainless steel autoclave that is connected with stirring and thermometer, add 220 gram tetrahydrofuran, 27.8 gram (0.2 mol) 2-amino-5-nitropyridine (Ⅱ), 0.5 gram zinc chloride, 27.0 gram (0.47 mol) S-propylene oxide, airtight autoclave, nitrogen replacement 3 times, 50 to 55 ℃ stirring reaction for 4 hours, cool to 20-25 ℃, transfer the reaction liquid to the 500 milliliter flask that is connected with stirring, thermometer and distillation device, The solvent was recovered by distillation, and the residue was recrystallized with methyl tert-butyl ether to obtain 47.7 g of N,N-bis(2S-2-hydroxypropylamino)-5-nitropyridine (Ⅲ1). The yield was 93.5%, and the liquid phase purity 99.7%.
Embodiment 2
[0076] Example 2: Preparation of N,N-bis(2R-2-hydroxypropylamino)-5-nitropyridine (Ⅲ2)
[0077] In the 500 milliliter stainless steel autoclave that is connected with stirring and thermometer, add 220 gram tetrahydrofuran, 27.8 gram (0.2 mol) 2-amino-5-nitropyridine (Ⅱ), 0.5 gram zinc chloride, 27.0 gram (0.47 mol) R-propylene oxide, airtight autoclave, nitrogen replacement 3 times, 50 to 55 ℃ stirring reaction 4 hours, cool to 20-25 ℃, transfer the reaction liquid to the 500 milliliter flask that is connected with stirring, thermometer and distillation device, The solvent was recovered by distillation, and the residue was recrystallized with methyl tert-butyl ether to obtain 47.6 g of N,N-bis(2R-2-hydroxypropylamino)-5-nitropyridine (Ⅲ2). The yield was 93.3%, and the liquid phase purity 99.8%.
Embodiment 3
[0078] Example 3: Preparation of (2S,6R)-2,6-dimethyl-4-(5-nitropyridin-2-yl)morpholine (IV)
[0079]In the 500 ml four-neck flask connected with stirring, thermometer, reflux condenser and variable head (connected with two constant pressure dropping funnels A and B), add 150 g of dichloromethane, 30.5 g (0.22 moles) of potassium carbonate, The constant pressure dropping funnel A is equipped with 25.5 grams (0.1 moles) of N, N-two (2S-2-hydroxypropylamino)-5-nitropyridine (Ⅲ1) and 50 grams of dichloromethane solution obtained by the method of embodiment 1, The constant-pressure dropping funnel B is equipped with a solution of 33.0 grams (0.12 moles) of trifluoromethanesulfonic anhydride and 50 grams of dichloromethane, cooled and kept between 15-20 ° C, and drop the two solutions at the same time for 1-2 hours After the addition is complete, thereafter, stir and react at 20 to 25°C for 4 hours, filter, add the resulting filtrate to 100 grams of water, adjust the pH value to 8-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| chromatographic purity | aaaaa | aaaaa |
| chromatographic purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com