Method for preparing sodium fluoride and co-producing calcium ammonium nitrate fertilizer by using ammonium fluoride
A technology using ammonium fluoride and calcium ammonium nitrate, applied in the directions of ammonium nitrate fertilizer, nitrate fertilizer, alkali metal fluoride, etc., can solve the problems of low solubility of sodium fluoride, high calcination temperature, and large environmental pollution, and achieve the solution The effect of small market capacity, low impurity content and high product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
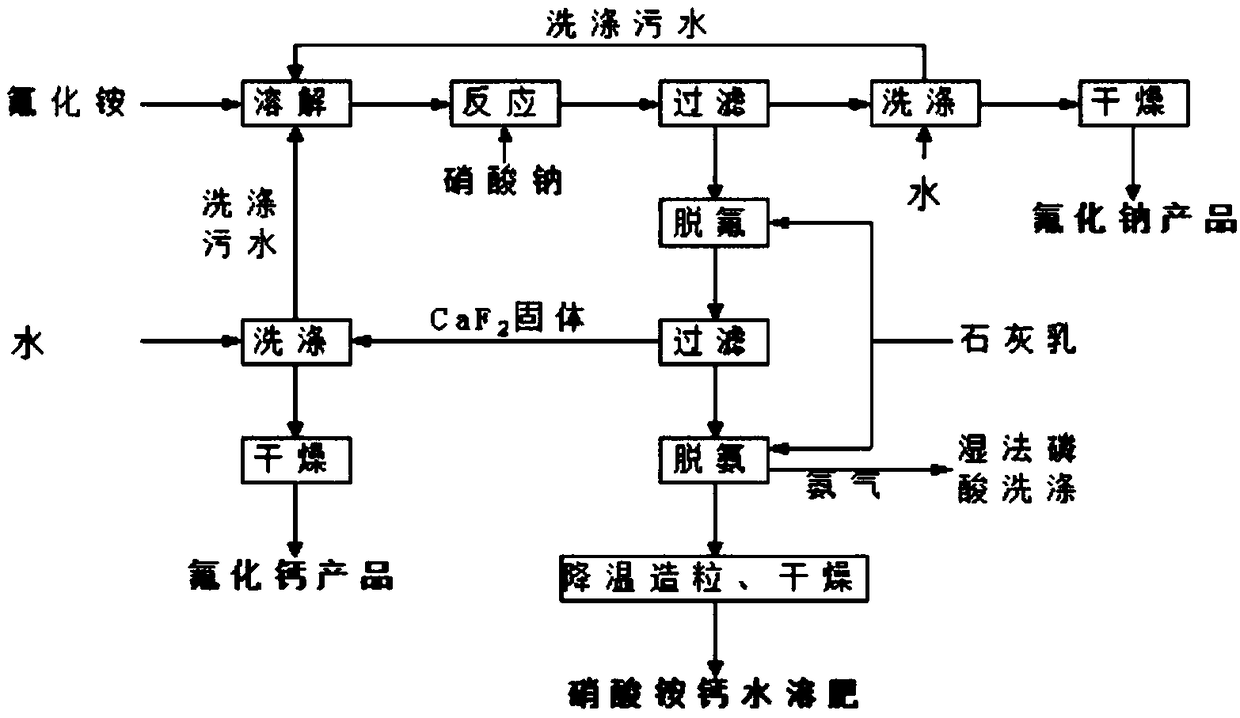
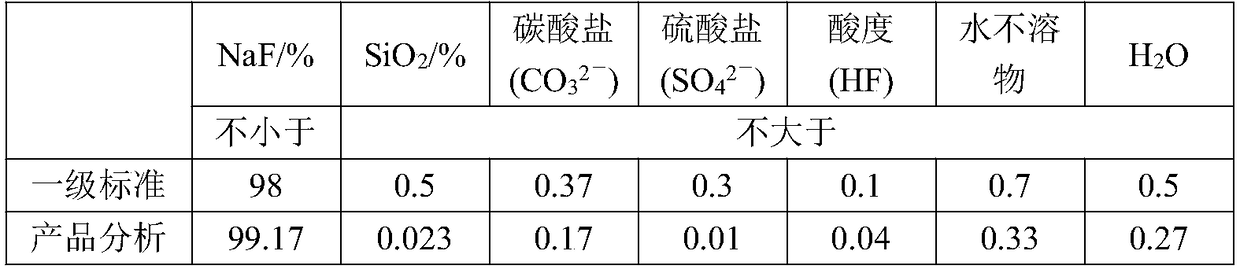
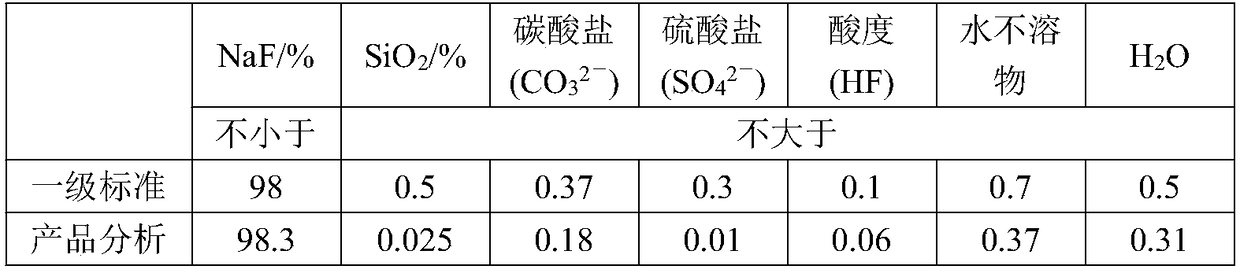
[0036] (1) Preparation of sodium fluoride: Dissolve 1000g of ammonium fluoride in water, control the concentration of ammonium fluoride to 40%, and the solution temperature to 40°C, then slowly add sodium nitrate to the solution to control the molar ratio n(NH 4 F): n(NaNO 3 ) was 0.85:1, 60r / min stirred and grown crystal for 1.25h, filtered the solid to obtain sodium fluoride solid, and collected the ammonium nitrate filtrate for later use;
[0037](2) defluorination of ammonium nitrate filtrate: the milk of lime added in the ammonium nitrate filtrate, the CaO content in the added lime milk is 9% of the ammonium fluoride content in the ammonium nitrate filtrate, the pH2 ) product, the defluorinated ammonium nitrate filtrate filtrate is collected for use;
[0038] (3) Preparation of calcium ammonium nitrate full water-soluble fertilizer: add lime milk to the defluorination filtrate, the molar content of CaO in the added lime milk is 46% of the molar content of ammonium nitrate...
Embodiment 2
[0044] (1) Preparation of sodium fluoride: Dissolve 1000g of ammonium fluoride in water, control the concentration of ammonium fluoride to 38%, and the solution temperature to 60°C, then slowly add sodium nitrate to the solution to control the molar ratio n(NH 4 F): n(NaNO 3 ) was 0.9:1, stirred and grown crystal for 0.5h, filtered the solid to obtain sodium fluoride solid, and collected the ammonium nitrate filtrate for later use;
[0045] (2) ammonium nitrate filtrate defluorination: the milk of lime added in the ammonium nitrate filtrate, the CaO content in the added lime milk is 8% of the ammonium fluoride content in the ammonium nitrate filtrate, control the pH2 ) product, the defluorinated ammonium nitrate filtrate filtrate is collected for use;
[0046] (3) Preparation of calcium ammonium nitrate fully water-soluble fertilizer: add milk of lime to the defluorination filtrate, the molar content of CaO in the added milk of lime is 47.5% of the molar content of ammonium ni...
Embodiment 3
[0052] (1) Preparation of sodium fluoride: Dissolve 1000g of ammonium fluoride in water, control the concentration of ammonium fluoride to 42%, and the temperature of the solution to be 20°C, then slowly add sodium nitrate to the solution to control the molar ratio n(NH 4 F): n(NaNO 3 ) was 0.8:1, stirred for 2 hours to grow crystals, filtered the solid to obtain sodium fluoride solid, and collected the ammonium nitrate filtrate for later use;
[0053] (2) ammonium nitrate filtrate defluorination: the milk of lime added in the ammonium nitrate filtrate, the CaO content in the added lime milk is 10% of the ammonium fluoride content in the ammonium nitrate filtrate, control the pH2 ) product, the defluorinated ammonium nitrate filtrate filtrate is collected for use;
[0054] (3) Preparation of calcium ammonium nitrate full water-soluble fertilizer: add milk of lime to the defluorination filtrate, the molar content of CaO in the added milk of lime is 45% of the molar content of a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com