A kind of monoterpene compound and its extraction method and application
An extraction method and compound technology, applied in the field of natural medicinal chemistry, can solve the problems of inability to cure, unsatisfactory treatment effect, poor drug efficacy, etc., and achieve good anti-hepatoma effect, significant curative effect, and significant anti-hepatitis B virus effect. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Characterization of Japopenoid A
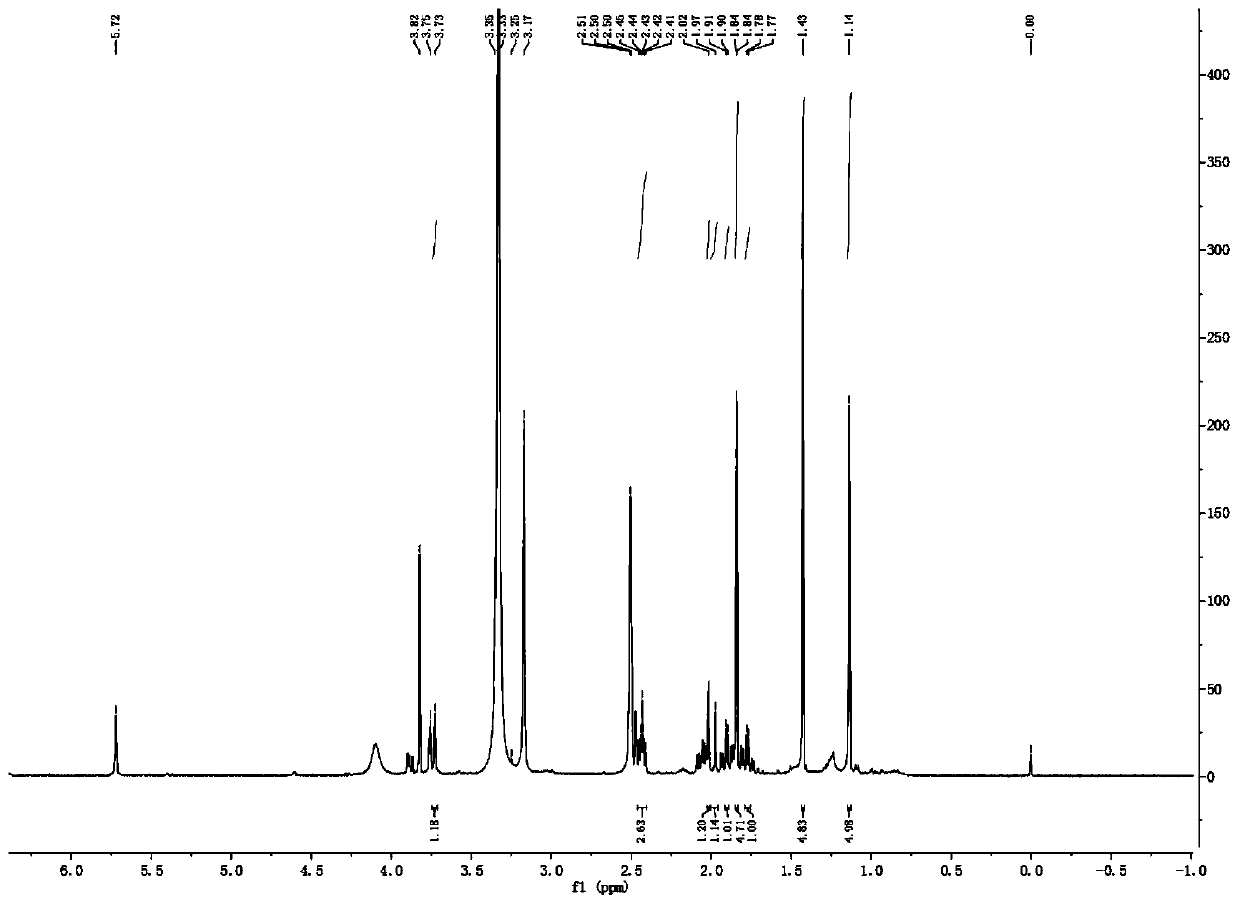
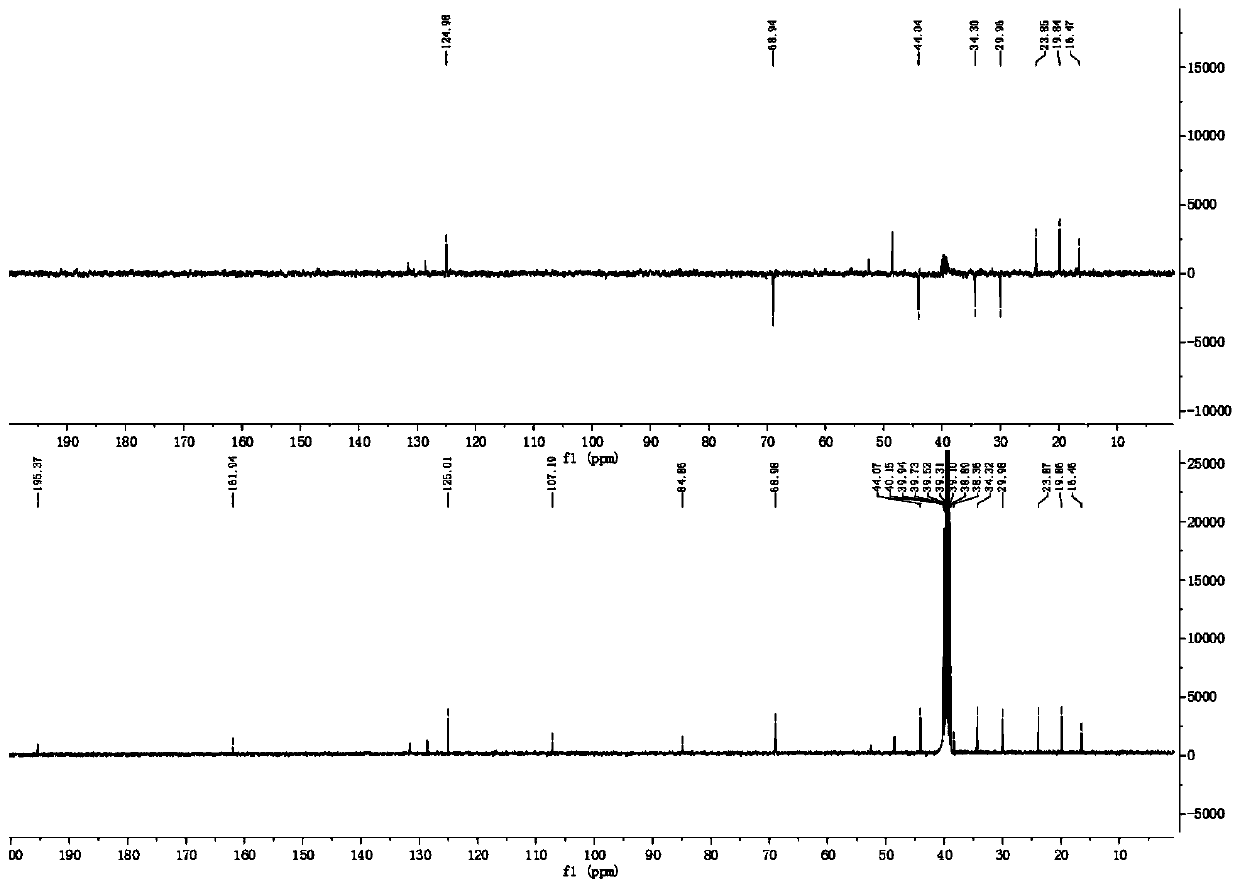
[0059] The new monoterpenoid compound provided by the present invention is light yellow oil, and HR-ESI MS (positive) gives quasi-molecular ion peak m / z 223.1324[M+H] + , suggesting that its molecular weight is 222; combined with hydrogen spectrum and carbon spectrum data (see Table 1 for details), it is determined that its molecular formula is C 13 h 18 o 3 , with a calculated unsaturation of 6.
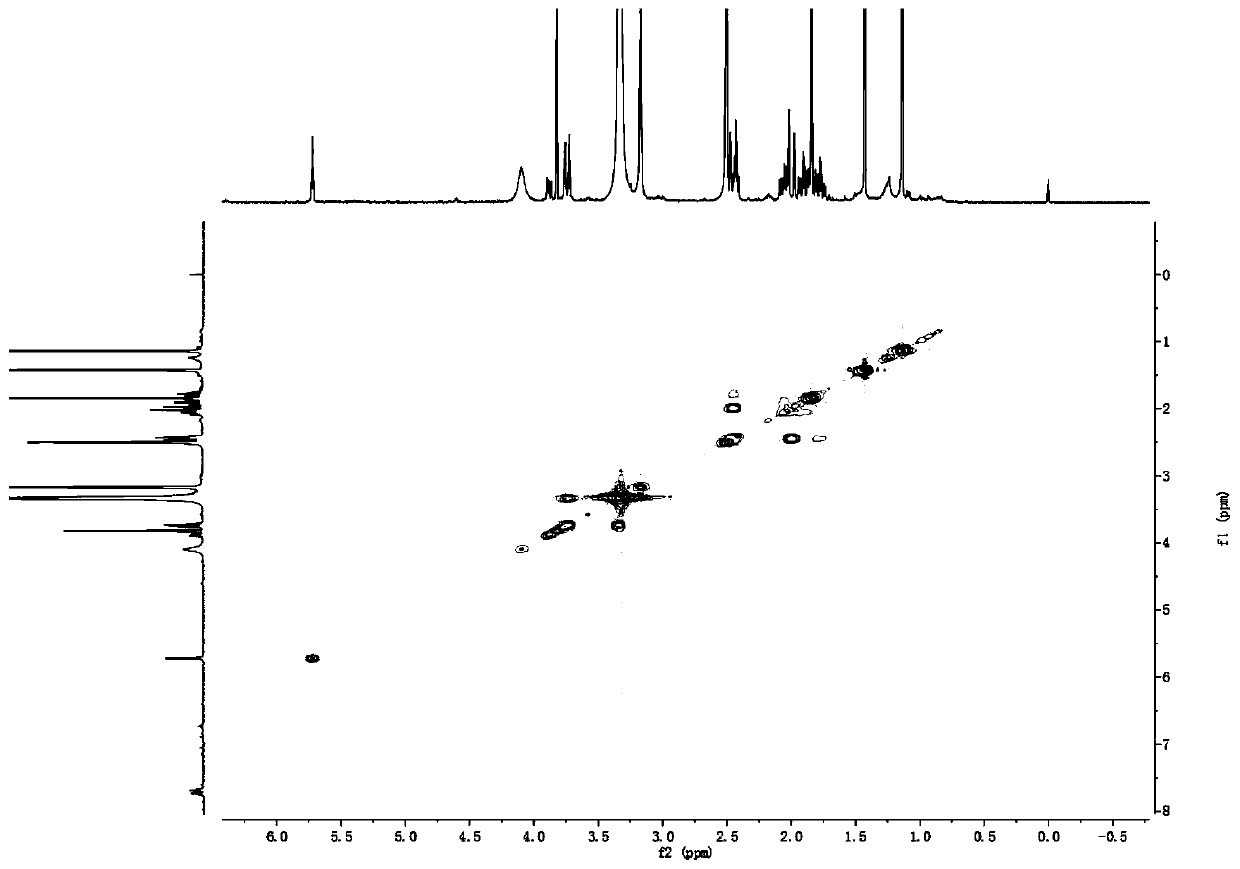
[0060] see Figure 1~6 , through analysis, it can be seen that the carbon spectrum of this monoterpenoid compound suggests that it contains 13 carbons, including three methyl groups, four methylene groups (wherein there is an oxygen connection), one methine group, five quaternary carbons (including a carbonyl, an alkene, and two oxycarbons). Moreover, the hydrogen spectrum and HSQC spectrum show that the compound contains 18 related proton hydrogen signals, which are three methyl [δ H 1.84(3H,s,H-11), 1.14(3H,s,H-12), and 1.43(3H,s,H-...
Embodiment 2
[0071] Preparation of Japopenoid A
[0072]At room temperature, 6.5kg honeysuckle (dried) was cold-soaked and percolated for 3 times with 75% ethanol, and the extracts were combined, then, the solvent was recovered under reduced pressure at 55°C, and concentrated to obtain 1500g of ethanol extract ; The ethanol extract is suspended in 3.5L water, then extracted three times with equal volumes of cyclohexane, ethyl acetate, n-butanol successively, respectively collect each extract and concentrate to obtain 130.3g cyclohexane respectively Alkane extract, 93.7g ethyl acetate extract and 199.8g n-butanol extract; get 83.0g of said ethyl acetate extract, dissolve with chloroform and methanol (10:1, 500ml), weigh 120g column chromatography Silica gel (100-200 mesh) is mixed sample, and another 1500g column chromatography silica gel (100-200 mesh) is weighed, and this silica gel is dissolved with dichloromethane and packed column, and equilibrate column with dichloromethane until the ...
Embodiment 3
[0078] Anti-tumor activity of Japopenoid A in vitro
[0079] The inventors also investigated the antitumor activity of the monoterpene compound Japopenoid A in vitro, and the tumor strains used in related experiments were liver cancer HepG2 cells and SMCC 7721 cells.
[0080] Specifically implement the MTT method: inoculate the cells in a 96-well cell culture plate, 200 μL per well (containing 2.5×10 4 tumor cells), at 37°C, 5% CO 2 In the incubator, and in the RPMI1640 medium containing 10%FBS, culture 24h; Add the monoterpenoid Japopenoid A of different concentration (200,100,50,25,12.5 and 6.25), continue to cultivate 48h; Add 20 μL of MTT (5 mg / mL) for 4 hours, and continue to store at 37 ° C, 5% CO 2 Incubate under the conditions for 4 hours, absorb the culture solution and add 150 μL of dimethyl sulfoxide, shake until the crystals are completely dissolved, and then detect the absorbance in a microplate reader, the detection wavelength is 570nm, the reference wavelength...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com