Low-heat-release and high-biological-activity compound bone cement and preparation method thereof
A bioactive, bone cement technology, applied in the direction of medical preparations with non-active ingredients, drug combinations, bone diseases, etc., can solve the problems of refractory degradation, short service life of PMMA bone cement, easy to become a source of infection, etc., to achieve good biological The effects of compatibility, excellent mechanical properties, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Example 1: Polymethyl methacrylate composite bone cement mixed with graphene and strontium-silver-hydroxyapatite
[0050] (1) 0.5 g of graphene and 1.18 mL of normal saline were used to prepare a graphene suspension by combining an ultrasonic cell pulverizer with an ultrasonic cleaner;
[0051] (2) Mix the above graphene suspension with 4.5 g strontium-silver-hydroxyapatite evenly, and mix it with 95 g polymethyl methacrylate, 38 mL methyl methacrylate, 0.02 mL hydroquinone , 0.8 mL N,N-dimethyl-p-toluidine, mix well, and record the temperature at 28 days of reaction with a thermometer, then inject 40×40×160 mm3 Put it in a triple mold with a temperature of 37 ± 1 ℃ and a humidity of 90% in a constant temperature and humidity curing box for curing until the specified age, and carry out compressive and flexural strength tests.
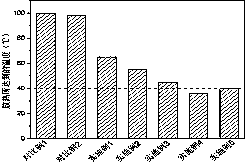
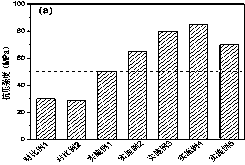
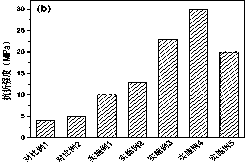
[0052] The temperature during the polymerization process is 65 °C; the compressive strength is 50 MPa, and the flexural strength is 10 MPa.
Embodiment 2
[0053] Example 2: Polymethyl methacrylate composite bone cement mixed with graphene and strontium-silver-hydroxyapatite
[0054] (1) 1 g of graphene and 1.18 mL of normal saline were used to prepare a graphene suspension by combining an ultrasonic cell pulverizer with an ultrasonic cleaner;
[0055] (2) Mix the above graphene suspension with 4 g strontium-silver-hydroxyapatite evenly, and mix it with 95 g polymethyl methacrylate, 38 mL methyl methacrylate, 0.02 mL hydroquinone , 0.8 mL N,N-dimethyl-p-toluidine, mix well, and record the temperature at 28 days of reaction with a thermometer, then inject 40×40×160 mm 3 Put it in a triple mold with a temperature of 37 ± 1 ℃ and a humidity of 90% in a constant temperature and humidity curing box for curing until the specified age, and carry out compressive and flexural strength tests.
[0056] The temperature during polymerization was 55 °C; the compressive strength was 65 MPa and the flexural strength was 13 MPa.
Embodiment 3
[0057] Example 3: Polymethyl methacrylate composite bone cement mixed with graphene and strontium-silver-hydroxyapatite
[0058] (1) 2 g of graphene and 1.18 mL of normal saline were used to prepare a graphene suspension by combining an ultrasonic cell pulverizer with an ultrasonic cleaner;
[0059] (2) Mix the above graphene suspension with 3 g strontium-silver-hydroxyapatite evenly, and mix it with 95 g polymethyl methacrylate, 38 mL methyl methacrylate, 0.02 mL hydroquinone , 0.8 mL N,N-dimethyl-p-toluidine, mix well, and record the temperature at 28 days of reaction with a thermometer, then inject 40×40×160 mm 3 Put it in a triple mold with a temperature of 37 ± 1 ℃ and a humidity of 90% in a constant temperature and humidity curing box for curing until the specified age, and carry out compressive and flexural strength tests.
[0060] The temperature during polymerization was 45 °C; the compressive strength was 80 MPa and the flexural strength was 23 MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com