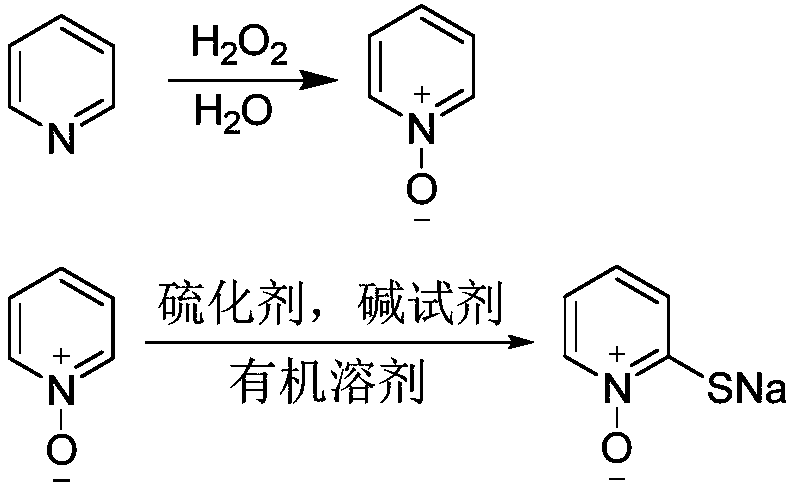
Novel synthetic method of sodium pyrithione
A technology of sodium pyridinethione and a synthesis method, applied in the field of synthesis of sodium pyridinethione, can solve the problems of low 2-chloropyridine reaction yield, low atom economy, large energy consumption for solvent recovery, and the like, and achieves economical efficiency. The effect of removing decolorization work, reducing synthesis cost, and high industrialization value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 23.85g of pyridine (0.30mol) and 1.43g of phosphomolybdic acid into a 250ml four-necked flask, add 20.00ml of water, install a spherical condenser and mechanical stirring, insert an internal temperature thermometer, then slowly raise the temperature to 80°C, dropwise Add 68.00g (0.60mol) of 30% hydrogen peroxide, and control the temperature at 80°C during the dropping process. After the dropwise addition, continue the reaction at 80°C, monitor the reaction by HPLC, stop the reaction when the raw material pyridine no longer decreases; the reaction solution Cool to room temperature, filter under reduced pressure, recover the catalyst, and distill the filtrate to remove water under reduced pressure to obtain 25.53 g of pyridine nitrogen oxide with a content of 95% (water content is 5%) and a yield of 85%.
[0040]Add 10.01g of pyridine nitrogen oxide (0.10mol), 110.57g of toluene (1.20mol) into a 250ml autoclave, then add 1.50g of sodium dodecylsulfonate, 37.74g (62%, 0...
Embodiment 2
[0042] Add 23.85g of pyridine (0.30mol) and 1.43g of phosphotungstic acid into a 250ml four-neck flask, add 20.00ml of water, install a spherical condenser and mechanical stirring, insert an internal temperature thermometer, then slowly raise the temperature to 80°C, dropwise Add 68.00g (0.60mol) of 30% hydrogen peroxide, and control the reaction temperature at 80°C during the dropwise addition process. After the dropwise addition, continue the reaction at 80°C, monitor the reaction by HPLC, and stop the reaction when the raw material pyridine no longer decreases. The solution was cooled to room temperature, filtered under reduced pressure, and the catalyst was recovered, and the filtrate was distilled off under reduced pressure to obtain 26.75 g of pyridine nitrogen oxide with a content of 96% (water content was 4%) and a yield of 90%.
[0043] Add 9.91g of pyridine nitrogen oxide (0.10mol), 110.57g of toluene (1.20mol) into a 250ml autoclave, then add 1.50g of sodium dodecyls...
Embodiment 3
[0045] Add 23.85g of pyridine (0.30mol) and 1.43g of phosphotungstic acid into a 250ml four-neck flask, add 20.00ml of water, install a spherical condenser and mechanical stirring, insert an internal temperature thermometer, then slowly raise the temperature to 80°C, dropwise Add 61.20g (0.54mol) of 30% hydrogen peroxide, and control the reaction temperature at 80°C during the dropwise addition process. After the dropwise addition, continue the reaction at 80°C, monitor the reaction by HPLC, and stop the reaction when the raw material pyridine no longer decreases. The solution was cooled to room temperature, filtered under reduced pressure, the catalyst was recovered, and the filtrate was distilled off under reduced pressure to obtain 27.34 g of pyridine nitrogen oxide with a content of 96% (water content was 4%) and a yield of 92%.
[0046] 9.91g pyridine nitrogen oxide (0.10mol), 106.18g p-xylene (1.00mol) are added in the 250ml autoclave, then add 1.50g sodium dodecyl diphen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com