Preparation method of (E, Z)-2, 4-ethyl decadienoate
A technology of ethyl decadienoate and octenoic acid, which is applied in the field of preparation of ethyl-2,4-decadienoate, can solve the problems of poor stability of phosphine ylide, difficulty in industrial amplification, generation of waste residue, etc. High temperature reaction conditions, simplification of operations, and the effect of improving reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
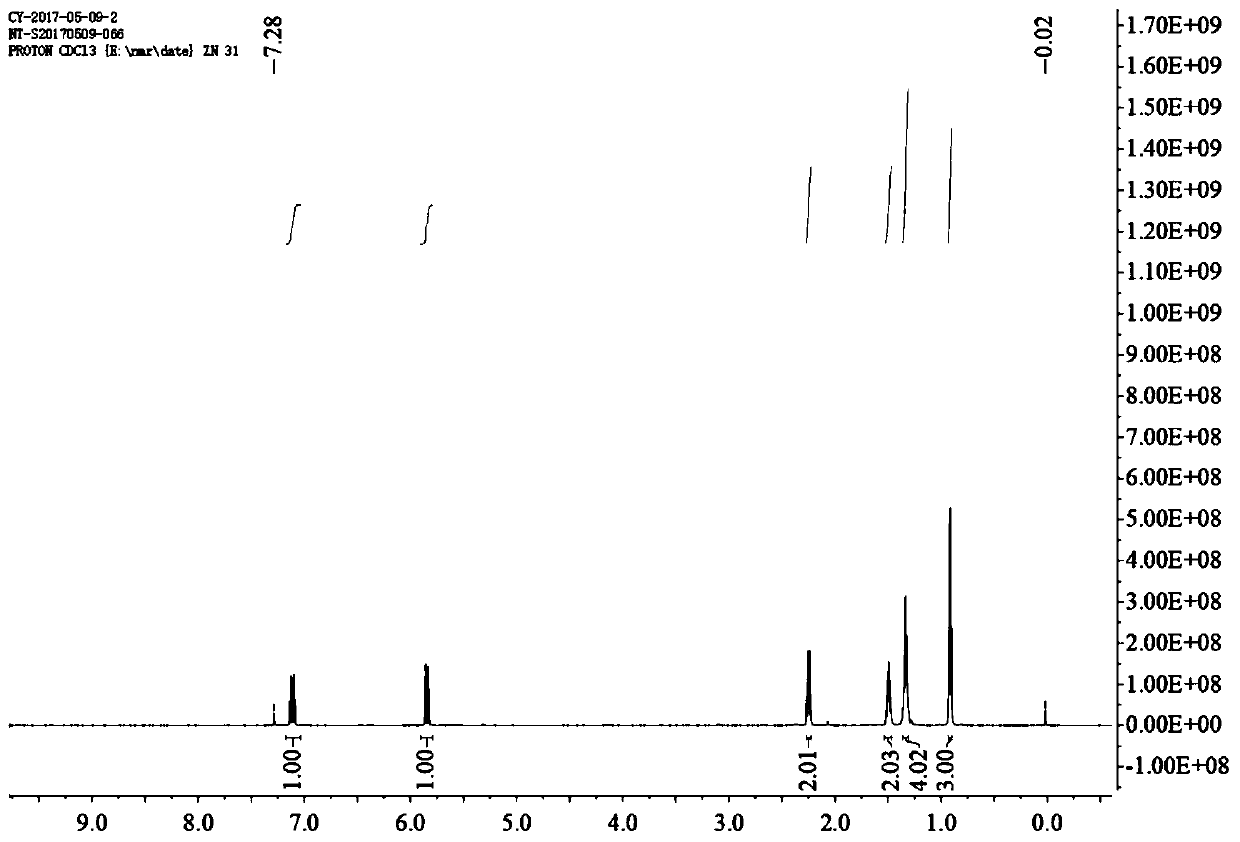
[0041]Synthesis of octenoic acid (compound 1): 250g of malonic acid and 400g of pyridine were mixed and placed in a 10L round-bottomed flask, the system was protected by argon replacement, and then 200g of n-hexanal was gradually added dropwise. After the addition, the system continued Stir the reaction at room temperature for 10 hours. After the reaction, add 3L of 20% phosphoric acid aqueous solution to the system in batches, continue stirring for 1 hour after the addition, and then extract the product with ethyl acetate, collect and combine the extract phases, and add Sodium sulfate water is dried, and after drying, ethyl acetate is removed by rotary evaporation to obtain 200g light yellow liquid, namely octenic acid (compound 1), and the 1H nuclear magnetic spectrum is shown in figure 1 , yield 71%.
Embodiment 2
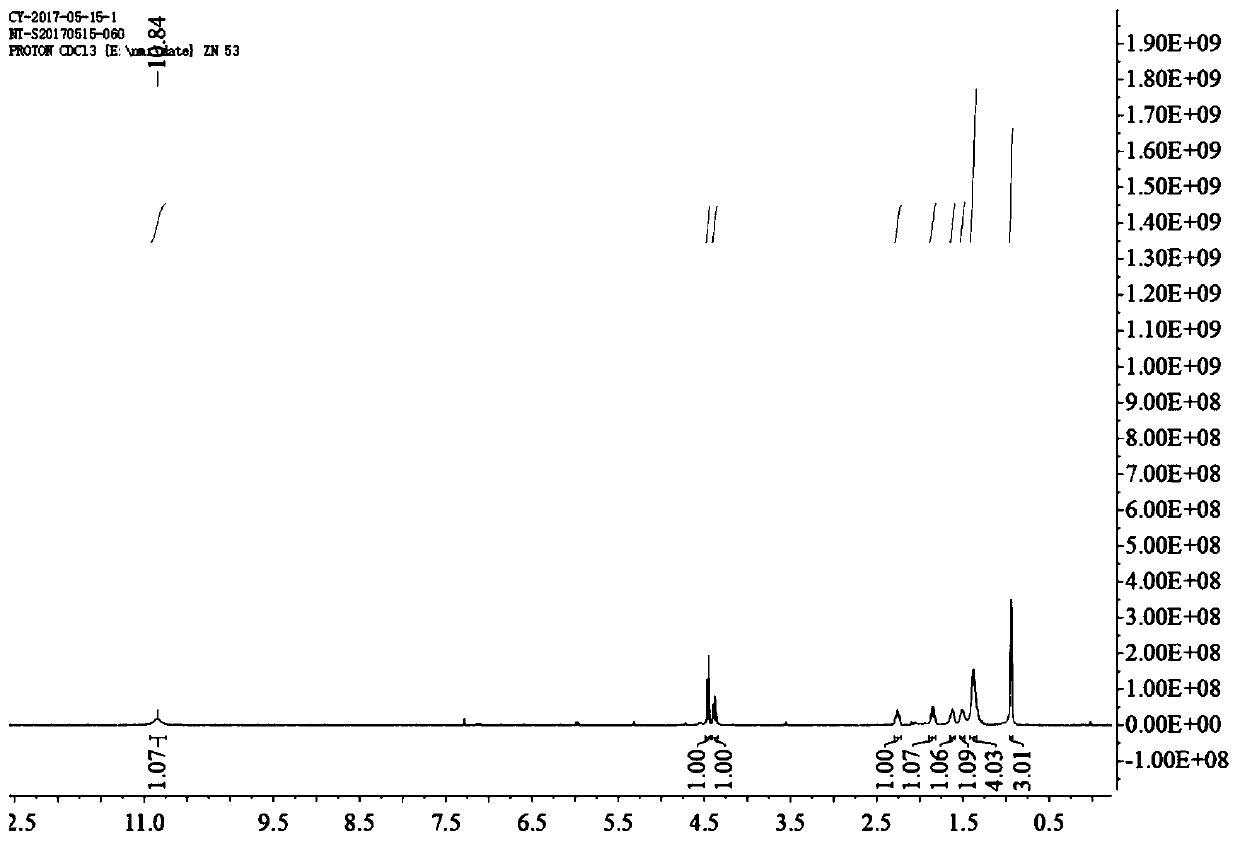
[0043] Preparation of 2,3-dibromooctanoic acid (compound 2): Weigh 50g of octenoic acid (compound 1) and dissolve it in 500mL of dichloromethane, then gradually add 64g of bromine dropwise, after the addition, the system is stirred at room temperature React for 5 hours. After the reaction, the system is orange-yellow and transparent. Then add 300 mL of 20% sodium bisulfite solution and stir for 10 minutes. The system becomes milky white and opaque. Extract with dichloromethane, collect and combine the extract phases, and add anhydrous sulfuric acid Sodium is dried, and after drying finishes, dichloromethane is removed by rotary evaporation, obtains 70g milky white oily substance, namely 2,3-dibromooctanoic acid (compound 2), and 1H NMR spectrum is shown in figure 2 , yield 66%.
Embodiment 3
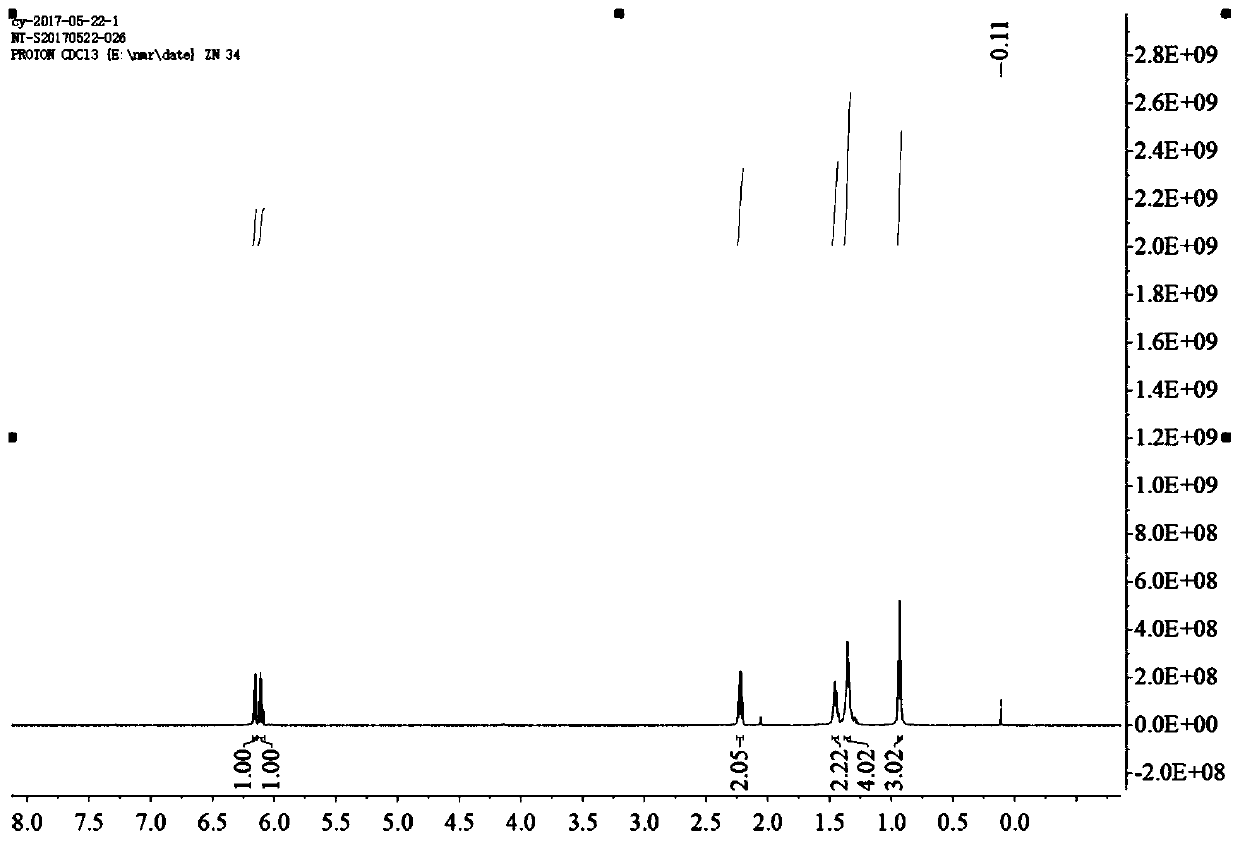
[0045] Preparation of 1-bromoheptene: Weigh 10g of 2,3-dibromooctanoic acid and dissolve it in 100mL of N,N-dimethylformamide, then add 3g of sodium bicarbonate, after the addition, the system reacts at 120°C for 2h, and the reaction ends Afterwards, the system was tea-yellow and transparent, and then most of the N,N-dimethylformamide in the system was removed by rotary evaporation, and the residue was diluted and dissolved with dichloromethane, and then washed with saturated sodium chloride solution, and then the organic phase, adding anhydrous sodium sulfate to dry, after drying, spin off the solvent to obtain a yellow oil, which was subjected to distillation to obtain 4g of bright yellow liquid, that is, 1-bromoheptene, 1H nuclear magnetic spectrum see image 3 , yield 67%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com