Method for preparing anlotinib hydrochloride
A technology of anlotinib hydrochloride and a synthesis method, applied in the field of medicine and chemical industry, can solve the problems of not greatly improving the yield of the docking step of chloroquinoline and phenol intermediate, high process cost, reducing route efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041]
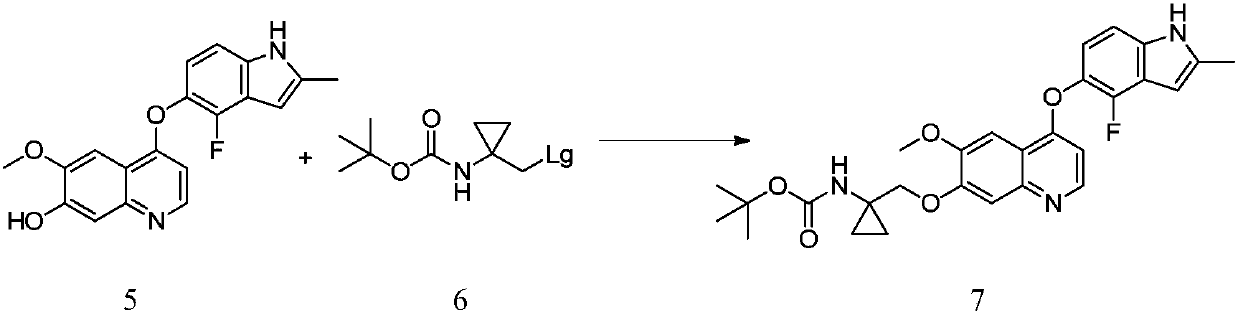
[0042] 7-benzyloxy-4-chloro-6-methoxyquinoline 1 (29.98g, 100mmol), 1-(2-fluoro-3-hydroxyl-6-nitrophenyl)propyl- 2-Kone 2 (21.32g, 100mmol) and N-methylpyrrolidone (150mL), were added with DABCO (11.22g, 100mmol), stirred evenly and then heated to 100°C to react overnight. After the reaction, water (600 mL) was added, and a large amount of solid precipitated out, which was filtered, and the crude product was recrystallized from a mixed solvent of isopropanol and water to obtain compound 3 (40.02 g, 84%).
[0043] MS(ESI)m / z=477.2[M+H] + , 1 H NMR (400MHz, DMSO-d6) δ8.57 (d, J = 5.2Hz, 1H), 8.08 (d, J = 8.4Hz, 1H), 7.51-7.59 (m, 4H), 7.49 (s, 1H) ,7.34-7.46(m,3H),6.74(d,J=5.2Hz,1H),5.32(s,2H),4.29(s,2H),3.94(s,3H),2.31(s,3H).
[0044] Here DABCO (triethylenediamine) organic base can be replaced by potassium carbonate, sodium carbonate, cesium carbonate, potassium tert-butoxide, sodium tert-butoxide, triethylamine, diisopropylethylamine, pyridine, DMAP, DBU.
Embodiment 2
[0046]
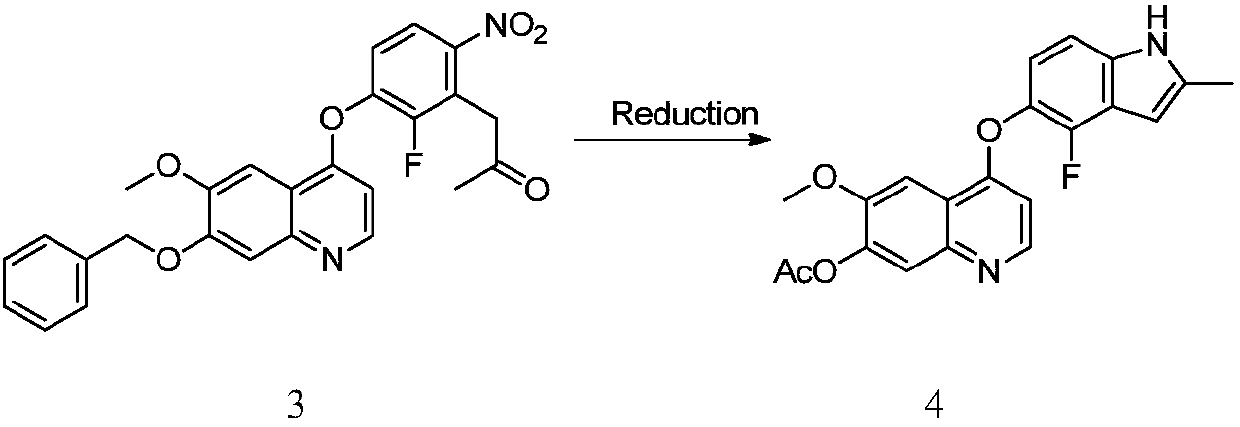
[0047]Add compound 3 (47.65g, 100mmol) to the hydrogenation bottle, add ethyl acetate (238mL) to dissolve, add sodium acetate (16.41g, 200mmol), add acetic anhydride (20.42g, 200mmol) and palladium carbon (5%, 2.14g ), switch the hydrogen gas three times under vacuum, pressurize to 0.15-0.20Mpa and keep the internal temperature at 50-55°C for 20-24 hours. At the end of the reaction, cool the diatomaceous earth to filter off the palladium carbon, concentrate and spin off part of the ethyl acetate, slowly add petroleum ether (238mL) to make a slurry, filter, wash with a small amount of petroleum ether, collect the solid and dry it to obtain compound 4 (35.37g, yield 93%) .
[0048] MS(ESI)m / z=381.2[M+H] + , 1 H NMR (400MHz, CDCl 3 )δ8.55(br,1H),8.49(d,J=5.2Hz,1H),7.80(s,1H),7.75(s,1H),7.07(d,J=8.4Hz,1H),6.92( t,J=8.0Hz,1H),6.44(d,J=5.2Hz,1H),6.36(s,1H),4.01(s,3H),2.47(s,3H),2.41(s,3H).
[0049] Here sodium acetate can be replaced by potassium bicarbonate, sodium...
Embodiment 3
[0051]
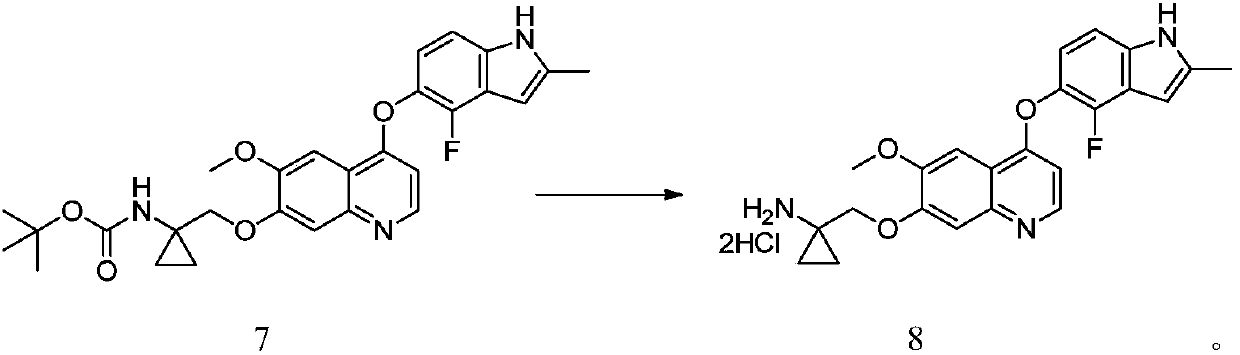
[0052] Compound 4 (38.04g, 100mmol) and isopropanol (190mL) were added into a three-necked flask, and potassium carbonate aqueous solution (15%, 95mL) was added, stirred evenly and heated to 40-45°C for 4-6 hours. At the end of the reaction, part of the isopropanol was spun off, and 5% dilute hydrochloric acid was added to adjust the pH to 4-5, beating for 1-2 hours, filtered, and the crude product was recrystallized with a mixed solvent of isopropanol and water to obtain compound 5 (30.79g, 91%) .
[0053] MS (ESI) m / z = 361.2 [M+Na] + , 1 H NMR(400MHz,DMSO-d6)δ11.41(br,1H),10.12(br,1H),8.37(d,J=5.2Hz,1H),7.58(s,1H),7.29(s,1H) ,7.21(d,J=8.8Hz,1H),6.99(t,J=8.0Hz,1H),6.24-6.30(m,2H),3.97(s,3H),2.42(s,3H).
[0054] Here potassium carbonate can be replaced by lithium hydroxide, sodium hydroxide, potassium hydroxide, sodium ethoxide, sodium methoxide, sodium tert-butoxide or potassium tert-butoxide, and isopropanol can be replaced by methanol, ethanol, tetrahydrofur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com