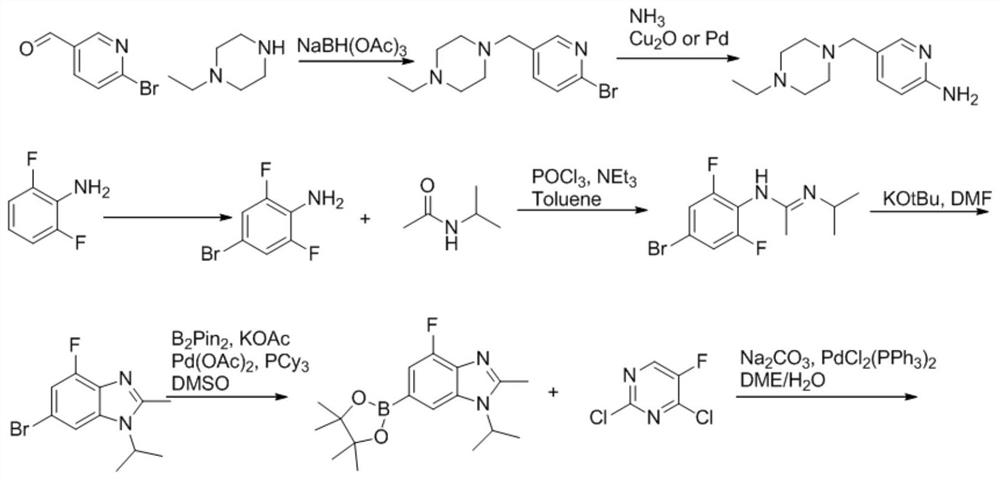
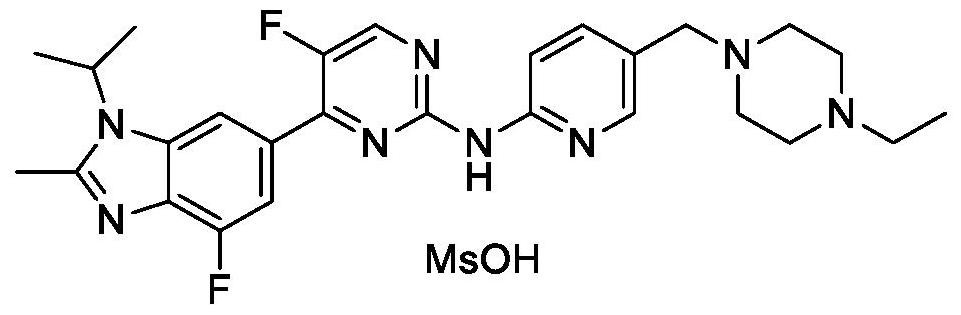
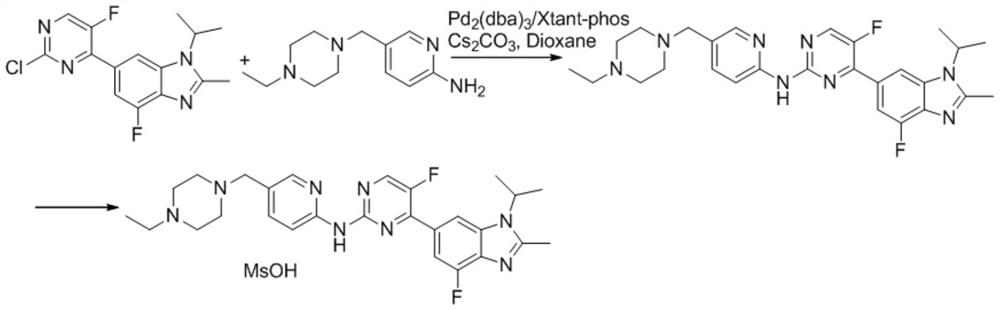
A kind of synthetic method of abemaciclib mesylate
A technology of mesylate and synthesis method, applied in the field of medicine and chemical industry, can solve the problems of multiple reaction yield, high environmental protection pressure, low yield and the like, and achieve the effects of avoiding precious metal catalyst, reducing route cost and high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046]
[0047] Add N-(4-bromo-2,6-difluorophenyl)acetamide compound 1 (25.00g, 100mmol), isopropylamine (6.50g, 110mmol), and acetonitrile (125mL) into the sealed reactor, and stir well. Add triethylamine (20.24g, 200mmol), increase the temperature to 90~95℃ and react for 8-10 hours. After the reaction, cool to room temperature, add water, spin off the acetonitrile and extract twice with dichloromethane (125mL). The combined organic phase was washed twice with saturated brine (125 mL), dried over sodium sulfate, filtered, and concentrated to obtain an oily compound 2 which was directly used for the next reaction.
[0048] In Example 1, potassium carbonate, sodium carbonate, sodium hydroxide, potassium hydroxide, potassium bicarbonate, diisopropylethylamine, 1,8-diazabicyclo[5.4.0] eleven carbon can be used for triethylamine. -7-ene (DBU) or triethylenediamine instead, the solvent acetonitrile can be replaced by dimethylformamide, dimethylacetamide, NMP or 1,4-dioxane.
Embodiment 2
[0050]
[0051] Compound 2 (100mmol, obtained from Example 1) and toluene (125mL) were added to a three-necked flask, p-toluenesulfonic acid (38.04g, 200mmol) was added, and the temperature was raised and refluxed for 16-24 hours. The reaction was cooled to room temperature and added Adjust the pH to 11-12 with 5% sodium hydroxide solution, separate the liquids, extract the aqueous phase with ethyl acetate (125mL) once, combine the organic phases and wash twice with saturated brine (125mL), dry with sodium sulfate, filter and concentrate to remove Part of the solvent was added with petroleum ether (125 mL), the solid was precipitated, and the slurry was filtered and dried to obtain compound 3 (21.42 g, two-step yield 79%).
[0052] In Example 2, p-toluenesulfonic acid can be replaced by hydrochloric acid, acetic acid, trifluoroacetic acid or fluoromethanesulfonic acid; the solvent toluene can be dichloromethane, 1,2-dichloroethane, 1,4-dioxane, tetrahydrofuran, Replace with ethyl...
Embodiment 3
[0054]
[0055] Add compound 3 (27.11g, 100mmol) and tetrahydrofuran (136mL) into a three-necked flask, stir to dissolve and cool to 0~5℃ in an ice-salt bath, switch nitrogen to the vacuum 3 times, and add 2.0M isopropylmagnesium chloride tetrahydrofuran solution (110mmol, 55.0mL), keep the internal temperature at 0~5℃ for 0.5~1 hour. Dissolve 2,4-dichloro-5-fluoropyrimidine (18.37g, 110mmol) in tetrahydrofuran (136mL) under the protection of nitrogen, add the catalyst iron triacetylacetonate (1.78g, 5mmol), stir evenly, the prepared Grignard The reagent solution was added dropwise to the reaction flask containing pyrimidine, and after the dropping, the temperature was raised to 55-60°C for 4-6 hours. After the reaction was completed, saturated aqueous ammonium chloride solution was added to quench the reaction. The mixture was extracted 3 times with ethyl acetate (216 mL), the combined organic phase was washed twice with water (216 mL), dried over sodium sulfate, filtered, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com