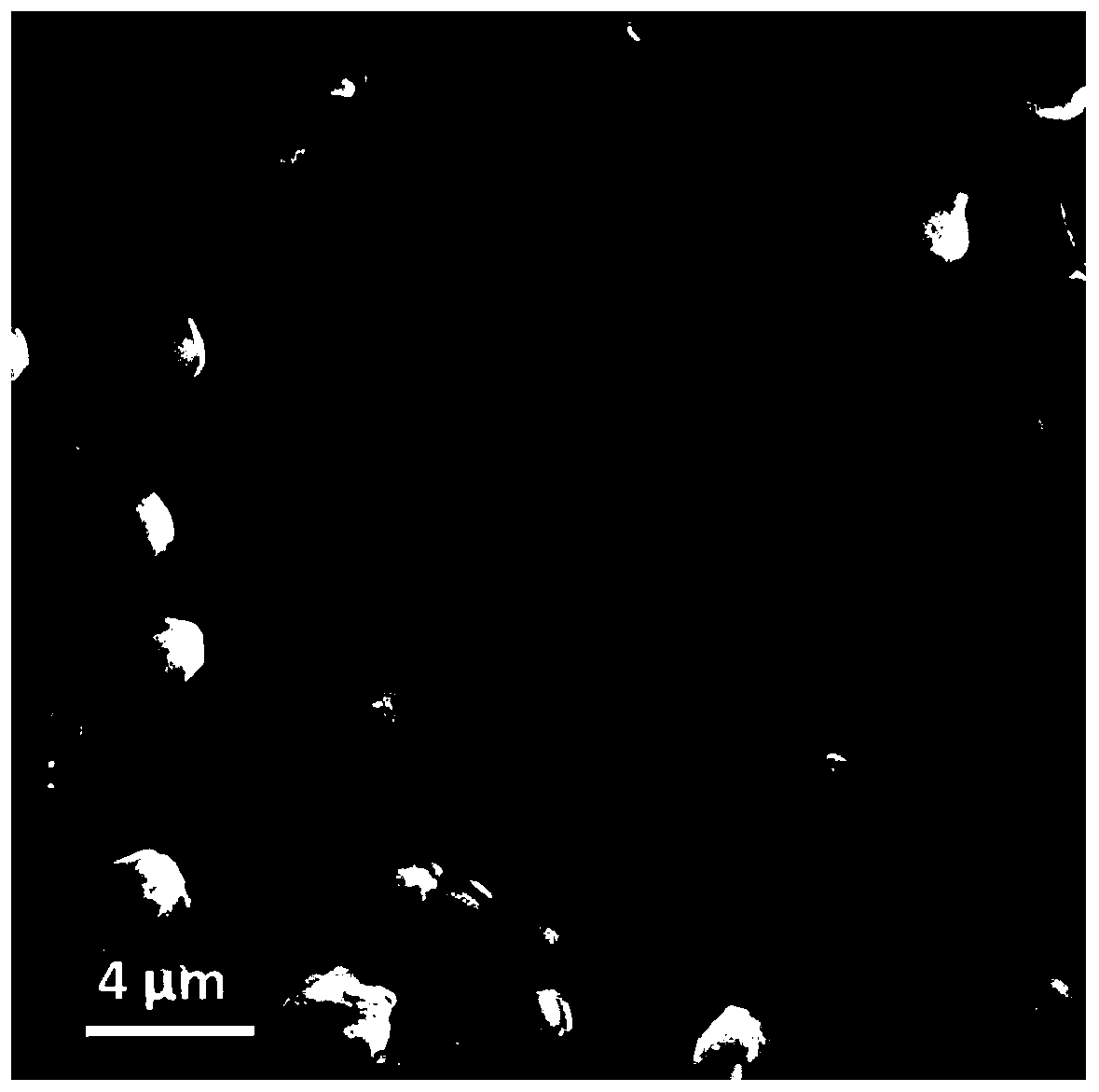
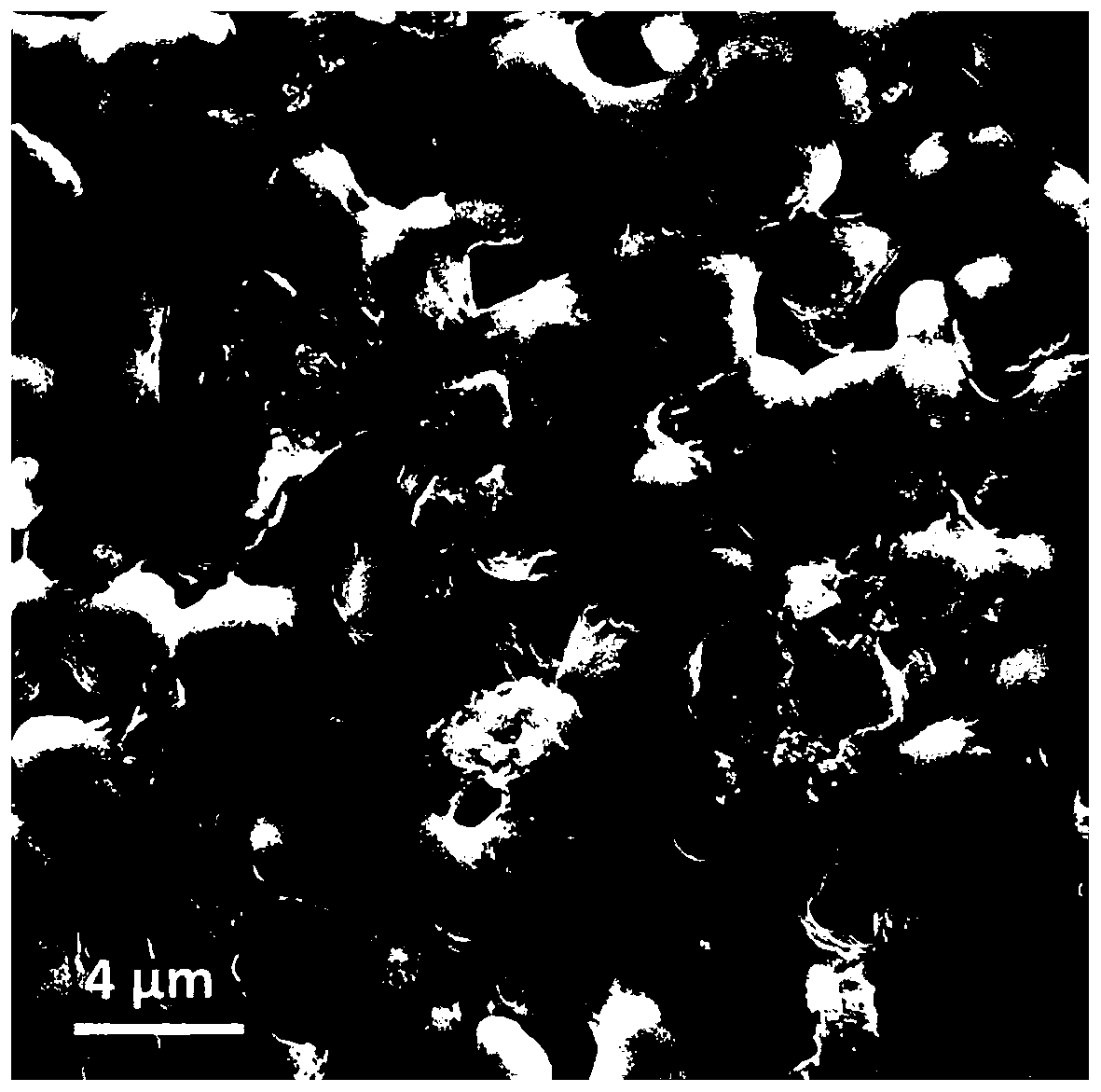
Composite solid electrolyte and preparation method thereof
A solid electrolyte and electrolyte technology, applied in non-aqueous electrolyte batteries, circuits, electrical components, etc., can solve the problems of poor wettability, easy breakage, fracture, and high interface impedance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0046] The invention relates to a composite solid electrolyte and a preparation method thereof. Firstly, a porous inorganic solid electrolyte ceramic green film is prepared and sintered to obtain a porous inorganic solid electrolyte frame internally connected with particles or fibers; and then the polymer-based solid electrolyte is The mixed solution is adsorbed into the framework of the porous inorganic solid electrolyte by vacuum; finally, the composite solid electrolyte is obtained by vacuum drying or in-situ polymerization, and the specific steps are as follows:
[0047] S1. Prepare a porous inorganic solid-state electrolyte frame by doctor blade coating or template method;
[0048] S101. Preparation of porous inorganic electrolyte framework by doctor knife coating method
[0049] S1011, uniformly mixing the solid electrolyte precursor powder, pore forming agent, dispersant, solvent, plasticizer and binder to obtain a mixed slurry; the mixing method is not limited, and met...
Embodiment 1
[0068] First, the Garnet-type solid electrolyte niobium-doped lithium lanthanum zirconium oxygen precursor was prepared by using the sol-gel method, and the required mass of lithium nitrate was weighed according to the stoichiometric ratio, and the lanthanum nitrate and zirconium oxynitrate were slowly added to deionized water; then A solution containing 2% niobium pentachloride is slowly added, and then citric acid is added to evaporate to dryness to obtain a xerogel; finally, a niobium-doped lithium lanthanum zirconium oxide precursor is obtained by high-temperature sintering.
[0069] Add 3 grams of niobium-doped lithium lanthanum zirconium oxide precursor, 0.2 grams of carbon spheres, some drops of triethanolamine and 9 grams of n-hexane / isopropanol azeotropic solvent, 0.8 grams of polyvinyl butyral and dibutyl phthalate The ester mixture was mixed and ball-milled for 5 hours at a rotation speed of 500 rpm to obtain a ball-milled mixed slurry.
[0070] The resulting slurry...
Embodiment 2
[0074] First, the sol-gel method is used to prepare the Garnet-type solid electrolyte niobium-doped lithium lanthanum zirconium oxygen precursor. The required mass of lithium nitrate is weighed according to the stoichiometric ratio, and the hexahydrate lanthanum nitrate and zirconium oxynitrate are slowly added to deionized water. Then slowly add a solution containing 2% niobium pentachloride, and then add citric acid to evaporate to dryness to obtain a xerogel; finally, sinter at a high temperature to obtain a niobium-doped lithium lanthanum zirconium oxide precursor.
[0075] Add 3 grams of niobium-doped lithium lanthanum zirconium oxide precursor, 0.2 grams of carbon spheres, some drops of triethanolamine and 9 grams of n-hexane / isopropanol azeotropic solvent, 0.8 grams of polyvinyl butyral and dibutyl phthalate The ester mixture was mixed and ball-milled for 4 hours at a rotation speed of 500 rpm to obtain a ball-milled mixed slurry.
[0076] The resulting slurry was tape-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com