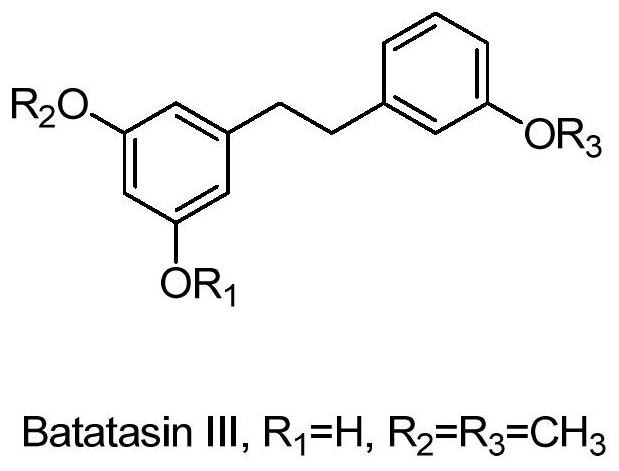
Application of yamcin Ⅲ in the preparation of medicines and cosmetics
A kind of yam element, product technology, applied in the direction of cosmetics, cosmetic preparations, ether preparation, etc., can solve the problems of pigment instability, skin damage, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
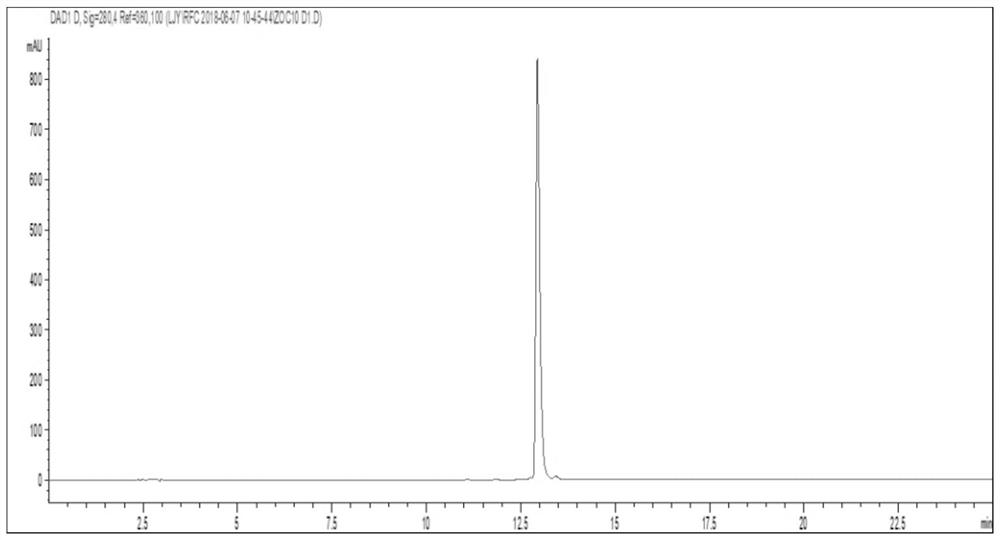
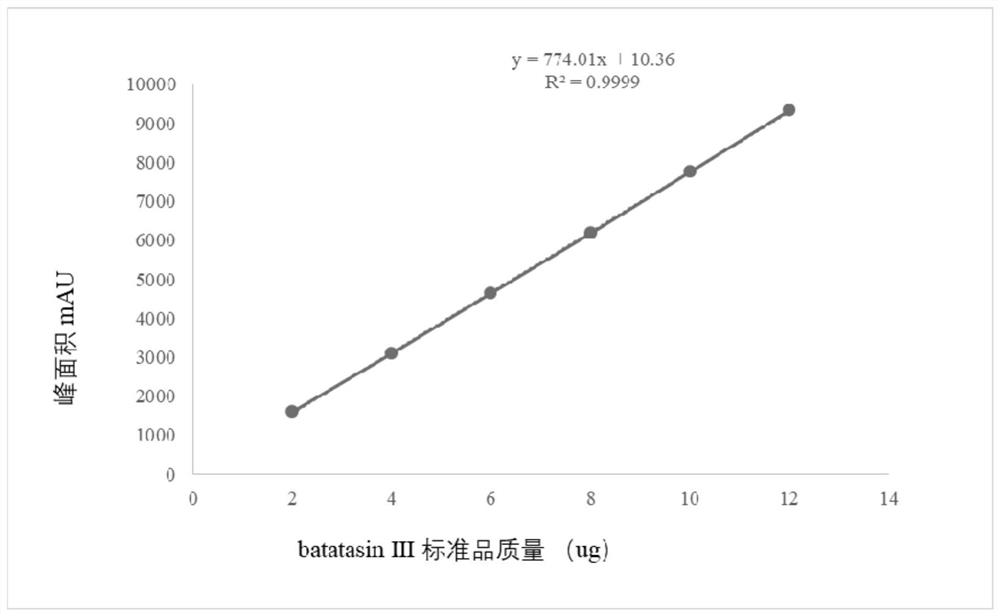
[0032] batatasinⅢorthogonal process
[0033] 1.1 Material test material: Bletilla striata medicinal material was produced in Hubei in July 2017, and was identified as a dried pseudobulb of Bletilla striata by the Kunming Institute of Botany, Chinese Academy of Sciences. The dried pseudobulbs of Baiji are crushed with a pulverizer and passed through a 40-mesh aperture sieve, sealed and stored for later use.
[0034] 1.2 Reagents: BatatasinⅢ standard products are self-made in the laboratory; absolute ethanol, methanol, and acetonitrile are all chromatographically pure, and are products of Sinopharm Chemical Reagent Company.
[0035] 1.3 Instruments Agilent 1260HPLC high performance liquid phase; KERN electronic analytical balance (Beijing Sartorius Instrument System Co., Ltd.); Heidolp rotary evaporator (German Hei-VAP company); chromatographic column
[0036] ZORBAX SB-C 18 (4.6×250mm, Agilent, USA).
[0037] 1.4 Test method
[0038] 1.4.1 Chromatographic conditions: using ...
Embodiment 2
[0058] After crushing 8.0 kg of white and dried tubers, soak them in ethanol (80%) at room temperature for 3 times, dissolve the concentrated extract under reduced pressure in water, and extract with ethyl acetate and n-butanol successively to obtain 220 g of ethyl acetate and n-butanol respectively 500g (obtain the active part extract, that is, white and extract). Ethyl acetate part (270g) silica gel mixed sample (200-300 mesh) was eluted by silica gel column chromatography with chloroform-methanol gradient (50:1-4:1), and was detected by TLC and merged into 9 components (Fr.A – I). Fr.A (60.9 g) was eluted with medium pressure ODS methanol-water (30% - 100%) to give 9 fractions. Fr.A2 (5.3g) was eluted with Sephadex LH-20 (chloroform-methanol 1:1), and then subjected to silica gel column chromatography, petroleum ether-acetone (3:1-1:1), to obtain the compound and the compound identified in the literature It is batatasin III (4.8 g).
Embodiment 3
[0060] Evaluation of whitening and anti-aging activities of whitening and anti-aging activities of whitening and extracts and its monomer compound batatasin III
[0061] 3.1 Experimental cells and reagents
[0062] Human dermal fibroblasts-adult (HDFa) were purchased from Cascade Biologics. DMEM (high glucose) medium, PBS, Hank's balanced salt solution (HBSS), penicillin and fetal bovine serum (FBS) were purchased from Hyclone Company; 0.25% trypsin (containing EDTA) was purchased from Gibico Company ; DPPH, water-soluble vitamin E (Trolox), mushroom tyrosinase, levodopa (L-Dopa) and kojic acid (KojicAcid) were purchased from Sigma Company; transforming growth factor β (transforming growth factor beta, TGF-β) Purchased from Peprotech Company; Collagen ELISA kit was purchased from TaKaRa Company; MTS reagent was purchased from Promega Company.
[0063] 3.2 Experimental method
[0064] 3.2.1 DPPH free radical scavenging experiment
[0065] Mix and react the drug to be tested...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com