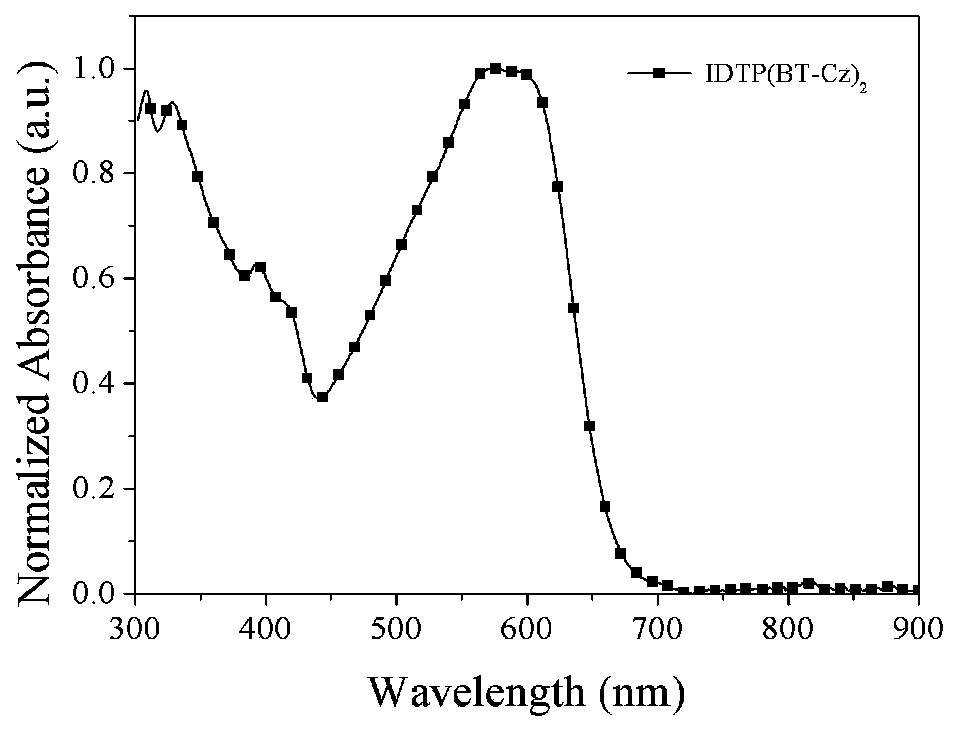
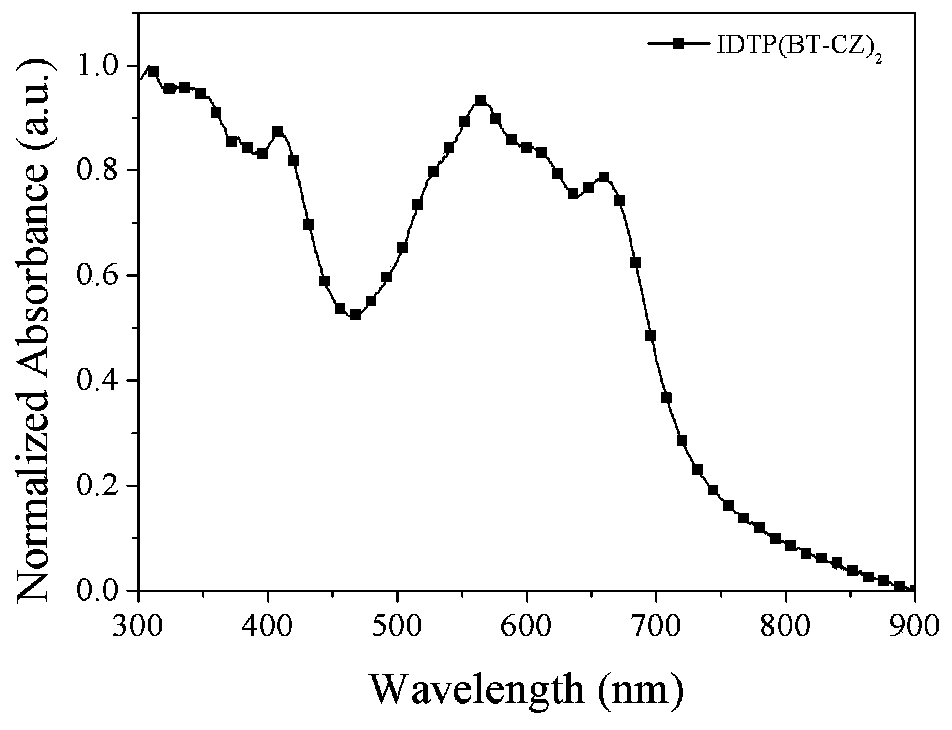
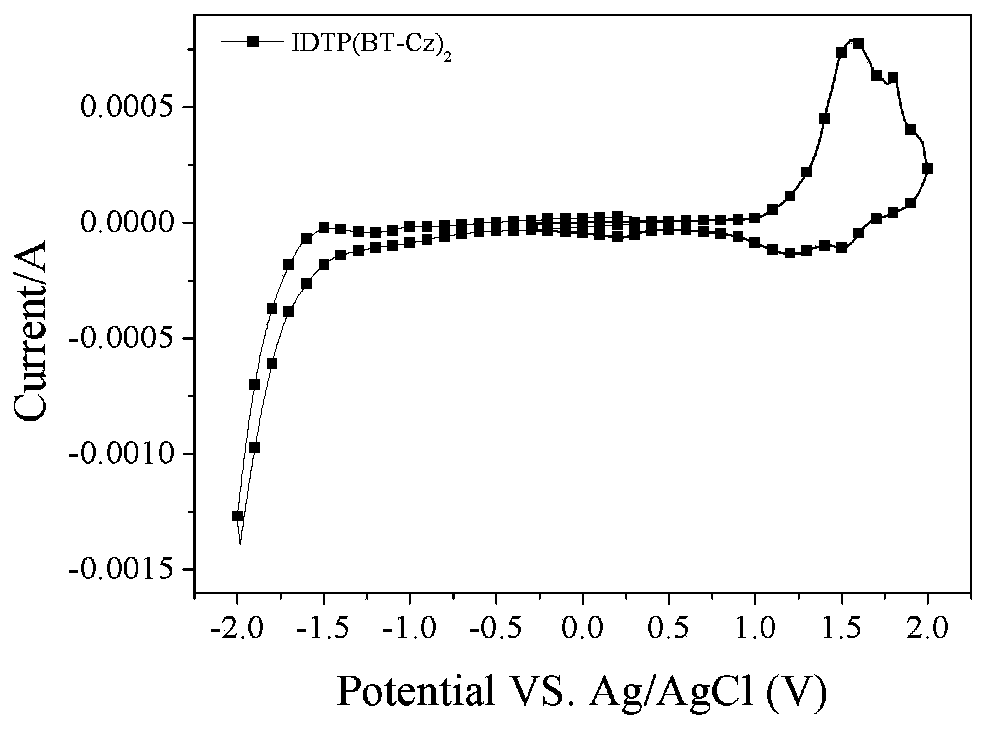
D(A-Ar)2-type conjugate compound based on dithiophene benzoisoaromatic heterocyclic fused ring and application of D(A-Ar)2-type conjugate compound
A technology of conjugated compounds and alkyl thiophene groups, applied in the field of organic small molecule photovoltaics, can solve the problems of complex device preparation, poor performance repeatability, and low service life, and achieve wide spectral band response, narrow band gap, and good film-forming performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056]5,5,10,10-Tetrakis(dodecyl)-7-(2-(thienyl)-5-hydro-dithiophene[3,2-c]isobenzopyran containing ditin substitution The synthesis of its synthetic route is shown in the figure below.
[0057]
[0058] 1.1 Synthesis of 2-(tributyltin)-3-methoxythiophene (1)
[0059] In a 250mL three-necked flask, dissolve 3-methoxythiophene (4.56g, 40mmol) in 80mL dry tetrahydrofuran, stir magnetically, and slowly add n-butyllithium (27.5mL, 1.6M) dropwise at -78°C, - React at 78°C for 0.5h, then react at room temperature for 2h. Put again at -78°C and add tributyltin chloride (14.3g, 44mmol) dropwise at once, and react at room temperature for 5h. The reaction solution was poured into 100 mL of water, extracted three times with petroleum ether, 30 mL each time, and the combined organic layers were washed with saturated brine three times, 50 mL each time. The organic layer was spin-dried under reduced pressure and dried in vacuo to obtain a pale yellow liquid (15.5 g, 98.0%). 1 HNMR (4...
Embodiment 2
[0071] Synthesis of 4-bromo-5,6-difluoro-7-(5-(9-octyl-9hydro-carbazole-3-)thiophene-2-)1,2,5-benzothiadiazole
[0072]
[0073] 2.1 Synthesis of 9-octyl-3-(thiophene-2-)-9-hydrogen-carbazole
[0074] Add 20mL of toluene, 3-bromo-9-octyl-9-hydrogen-carbazole (1.5g, 4.19mmol), 2-thiophene tributyltin (1.88g, 5.03mmol), bis(triphenyl Phosphine)palladium dichloride (88mg, 0.13mmol). Stir and heat to 110° C. under a nitrogen atmosphere, stop the reaction after 12 h, and cool to room temperature. The solvent was removed by rotary evaporation, and the mixed solution of petroleum ether / dichloromethane with a volume ratio of 5:1 was used as the eluent for column chromatography to obtain 1.36 g of the product with a yield of 90.0%. 1 HNMR (400MHz, CDCl 3 )δ8.32(s,1H),8.14(d,J=8.0Hz,1H),7.73(dd,J=8.5,1.8Hz,1H),7.50–7.45(m,1H),7.44–7.37(m ,2H),7.34(dd,J=3.5,1.1Hz,1H),7.26(d,J=1.1Hz,1H),7.11(dd,J=4.0,4.0Hz,1H),4.30(t,J= 8.0Hz, 2H), 1.88(m, 2H), 1.64(m, 2H), 1.35(m, 10H), 0.93(t, J...
Embodiment 3
[0080] Synthesis of 4-bromo-5,6-difluoro-7-(5'-octyl-[2,2'-dithiophene]-5-)1,2,5-benzothiadiazole
[0081]
[0082] Add 20mL of toluene, (5'-octyl-[2,2'-dithiophene]-5-)tributyltin chloride (1.72mg, 3.04mmol), 4,7-dibromo- 5,6-Difluoro-2,1,3-benzothiadiazole (1.0 g, 3.04 mmol), bis(triphenylphosphine)palladium dichloride (64 mg, 0.09 mmol). Stir and heat to 80°C under nitrogen atmosphere, stop the reaction after 4h, and cool to room temperature. The solvent was removed by rotary evaporation, and the mixed solution of petroleum ether / dichloromethane with a volume ratio of 5:1 was used as the eluent for column chromatography to obtain 271 mg of the product with a yield of 89.1%. 1 HNMR (CDCl 3 ,400MHz)δ8.19(d,J=4.0Hz,1H),7.24(dd,J=4.0,1.3Hz,1H),7.16(d,J=4.0Hz,1H),6.74(d,J=4.0 Hz,1H),2.82(t,J=8.0Hz,2H),1.70(dt,J=15.2,7.7Hz,2H),1.27(m,10H),0.89(t,J=8.0Hz,3H).
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com