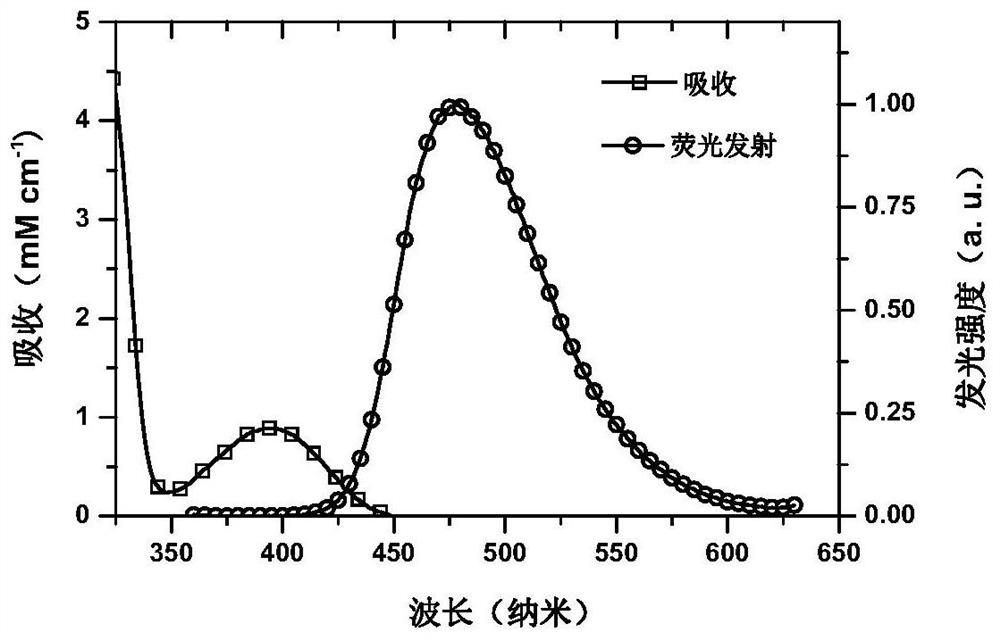
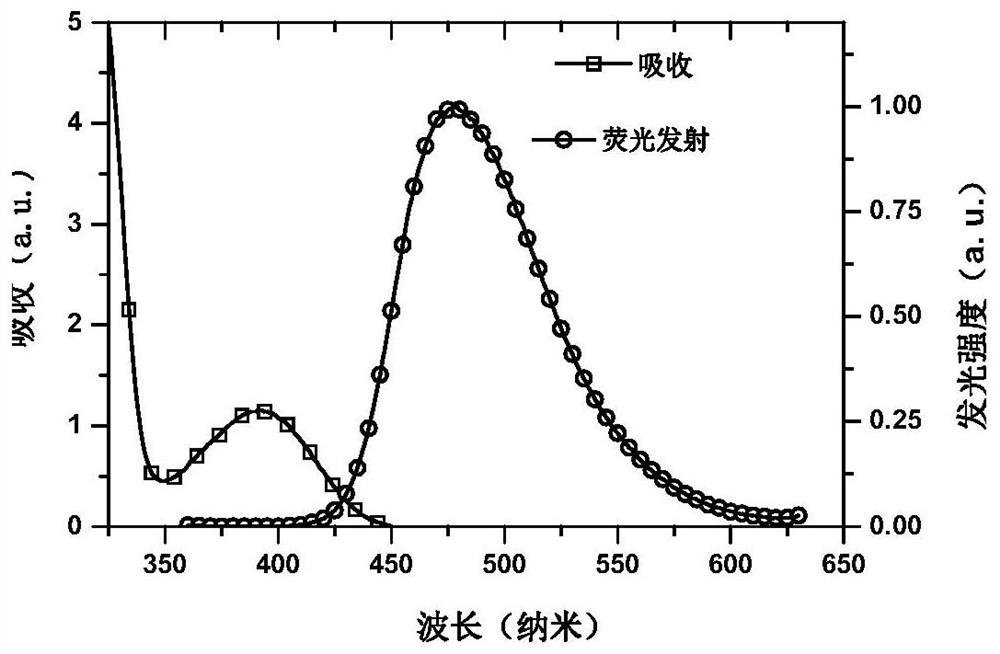
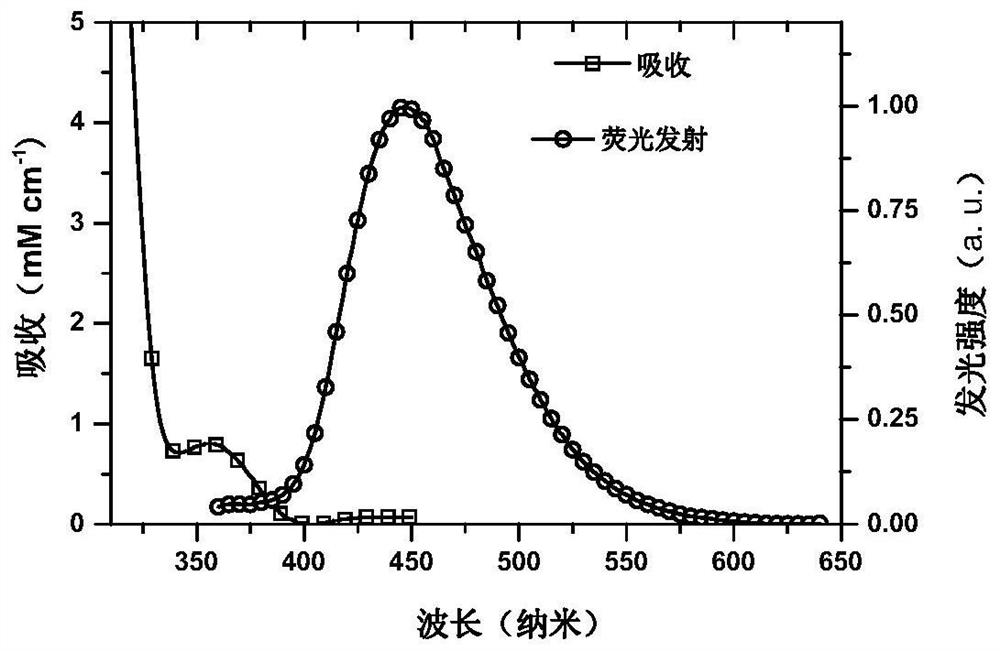
A small molecule luminescent material based on triple spiro acridine donor unit and its preparation method and application
A triple-spiro acridine, luminescent material technology, applied in luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., can solve problems such as lack of thermally activated delayed fluorescent materials, etc. Output coupling constant, good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of a non-conjugated end group containing a heteroatom sulfur-containing triple spiro acridine donor unit (a) of this embodiment:
[0059] (1) Preparation of intermediate 1-1:
[0060] After adding (2-bromophenyl)(phenyl)sulfane (5.3 g, 20 mmol) into a 250 mL three-necked flask, 150 mL of dry tetrahydrofuran was added. The mixture solution was cooled to -78°C and then degassed for 20 minutes. After that, 8.8 mL of n-butyllithium (2.5 mmol mL -1 ), kept stirring at -78°C for 1 hour, dissolved anthracene-9,10-dione (3.33g, 16mmol) in 50mL of anhydrous tetrahydrofuran, and added dropwise to the above low-temperature solution with a syringe. After the dropwise addition, continue to stir at this temperature for 1 hour, then raise the temperature to room temperature and stir for 4 hours. Then the mixture solution was treated with 5mL methanol and 10mL dilute hydrochloric acid (1mmolmL -1 ) was quenched, and then 100 ml of deionized water was added. And extract...
Embodiment 2
[0076] The preparation of a non-conjugated end group containing fluorene unit triple spiro acridine donor unit (b) of this embodiment:
[0077] (1) Preparation of intermediate 2-1:
[0078] After adding 2-bromo-1,1'-biphenyl (4.7 g, 20 mmol) into a 250 mL three-necked flask, 150 mL of dry tetrahydrofuran was added. The mixture solution was cooled to -78°C and then degassed for 20 minutes. After that, 8.8 mL of n-butyllithium (2.5 mmol mL-1 ), kept stirring at -78°C for 1 hour, dissolved anthracene-9,10-dione (3.33g, 16mmol) in 50mL of anhydrous tetrahydrofuran, and added dropwise to the above low-temperature solution with a syringe. After the dropwise addition, continue to stir at this temperature for 1 hour, then raise the temperature to room temperature and stir for 4 hours. Then the mixture solution was treated with 5mL methanol and 10mL dilute hydrochloric acid (1mmolmL -1 ) was quenched, and then 100 ml of deionized water was added. And extracted three times with dich...
Embodiment 3
[0094] Preparation of a triple-spiro acridine donor unit (c) whose end group contains a non-conjugated sulfone group in this embodiment:
[0095] The reaction equation is as follows:
[0096]
[0097] Intermediate (a) (1.05 g, 2 mmol) was dissolved in 150 ml of glacial acetic acid and purged with nitrogen for 20 minutes. Finally, the mixture solution was heated to 80° C., stirred for 10 minutes, and then 5 ml of hydrogen peroxide was added. The reaction was carried out at this temperature for 8 hours. The reaction was stopped, and the glacial acetic acid solvent was distilled off under reduced pressure. Column chromatography petroleum ether / dichloromethane (2:1) obtained a light yellow solid EI-MS (m / z): calcdfor C 38 h 25 NSO 2 ; Molecular weight: 559.16; found: 559.32, [M + ].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com