Coxsackie A16 virus IGA antibody quantum dot immunofluorescence chromatography test strip and kit
A chromatographic test strip and immunofluorescence technology, applied in the field of microbial detection, can solve the problems of fear of blood collection, interfere with the work of medical staff, etc., and achieve the effects of easy control, avoidance of spread and high fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of Coxsackie A16 virus IgA antibody quantum dot immunofluorescence chromatography test strip
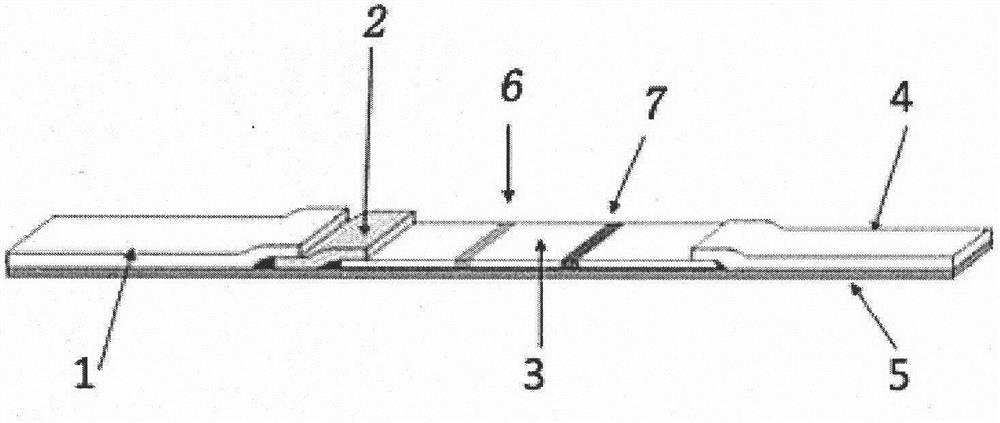
[0031] 1. Preparation of quantum dot particle labeling pad
[0032]Take 10-50 μl of water-soluble CdTe / ZnSe core-shell quantum dots (surface carboxyl modification, Beijing Zhongke Wuyuan Biotechnology Co., Ltd., article number W-3006-570), centrifuge at 6000rpm for 5-10min, add 100μl 10mM PBS (pH 7.2) Dissolved to obtain a quantum dot solution. Add 10-50 μl of 2% (mass concentration) N-hydroxysuccinimide and 2% (mass concentration) 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride to the quantum Add 100-1500 μl of goat anti-human IgA antibody (Sigma, Cat. No. 10884) solution at a concentration of 5 mg / ml to the spot solution, and stir at room temperature for 1-6 hours. After the reaction is complete, centrifuge at 6000 rpm for 5-10 min to obtain a precipitate, and add 1% (mass volume concentration) BSA (bovine serum albumin) solution to the p...
Embodiment 2
[0041] Embodiment 2: Specimen test and result judgment
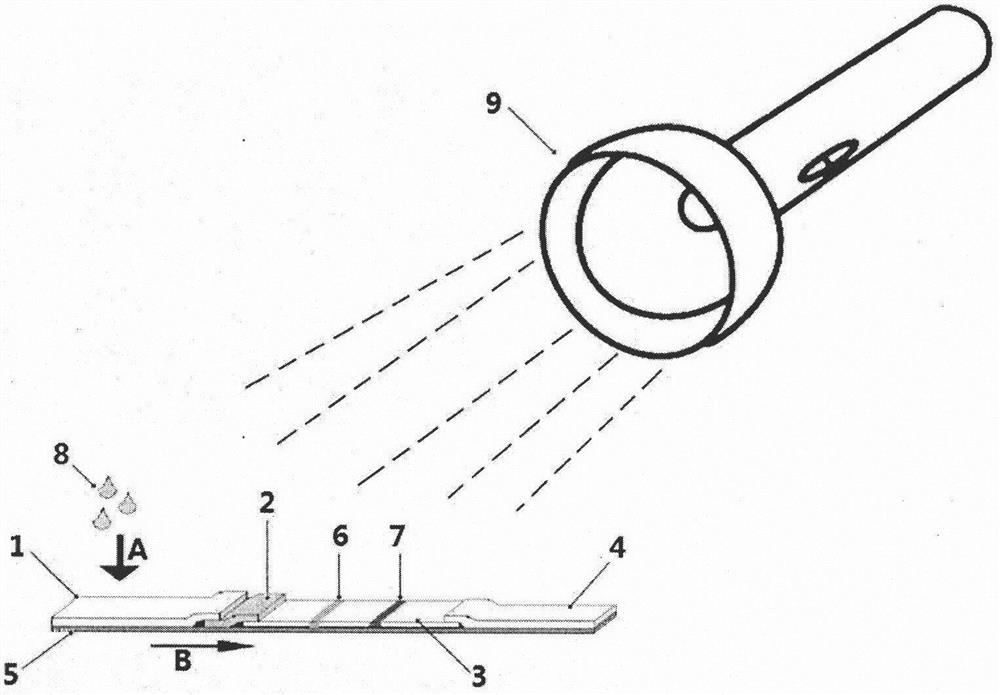
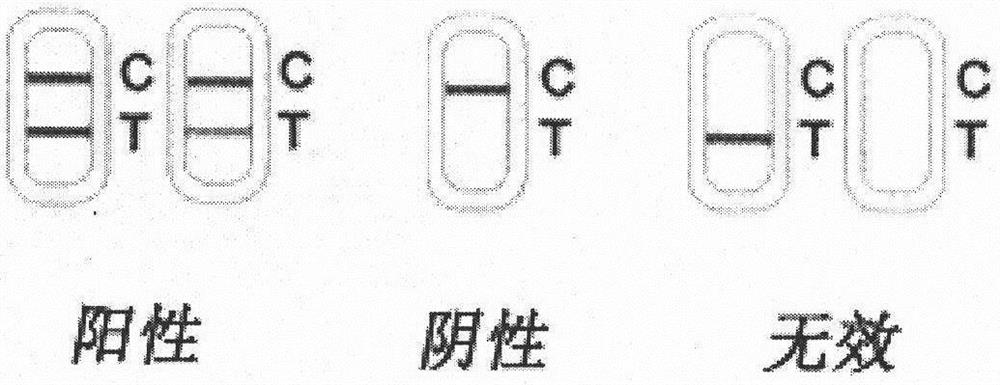
[0042] Put the cotton swab into the subject's mouth, collect saliva on the cheeks and under the tongue, put the collected cotton swab into the sample tube containing the sample diluent and squeeze it fully to obtain sample solution 8, suck 100-200μl The sample solution 8 is dropped on the sample pad 1 of the immunofluorescence chromatography test strip of the present invention along the direction A, and the sample solution 8 moves toward the absorbent paper along the chromatography direction B. Irradiate the test strip with the ultraviolet flashlight 9 for 20-30 minutes to observe the result. The wavelength range of ultraviolet light is 200-400nm.
[0043] The formula for the sample diluent is as follows:
[0044] 0.3% gelatin, 0.15% casein, 0.1% tryptone, 0.1% ProClin300, 10 mM PBS, pH 7.2.
[0045] figure 2 A schematic diagram of the detection process of the Coxsackie A16 virus IgA antibody quantum dot immunofluor...
Embodiment 3
[0047] Embodiment 3: Comparison of the results of the Coxsackie A16 virus IgA antibody quantum dot immunochromatography test strip and the fluorescent PCR method of the present invention
[0048] Screen 100 clinical patients with hand, foot and mouth disease, collect saliva samples and throat swab samples from the patients, and use the Coxsackie A16 virus IgA antibody quantum dot immunochromatography test strip diagnostic method and the Coxsackie virus A16 virus fluorescent PCR method respectively. The test results are shown in Table 1 below:
[0049] Table 1: The results of the comparison between the diagnostic method of quantum dot detection test strip and fluorescent PCR method for IgA antibody of Coxsackie virus A16 virus
[0050]
[0051] Thus, the Coxsackie A16 virus IgA antibody quantum dot immunochromatographic test strip of the present invention is compared with the fluorescent PCR method, the positive rate reaches 94.59%, and the specificity is 92.06%, which can m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com