Synthetic method of flavonoid compound
A technology of flavonoid compounds and synthetic methods, which is applied in the field of organic synthesis, can solve the problems of aroyl chlorides being sensitive to water vapor, easy to deteriorate and transform, and achieve the effects of convenient process, mild reaction conditions and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
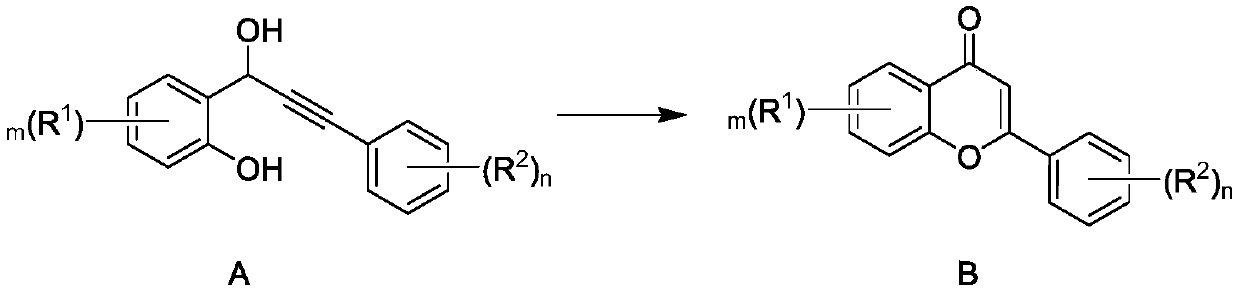
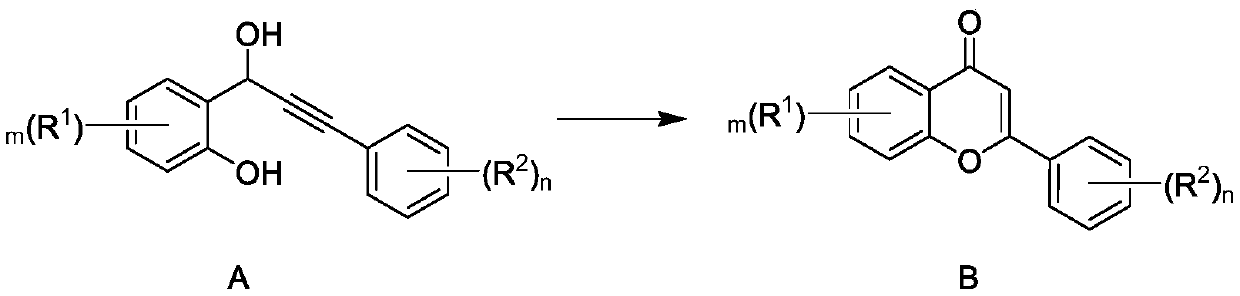
[0027] The invention provides a synthesis method of flavonoid compounds. In the synthesis method, high-activity catalyst Co-NC is selected as a catalyst, and oxygen is used as an oxidant to realize efficient and high-selectivity synthesis of flavonoid compounds.
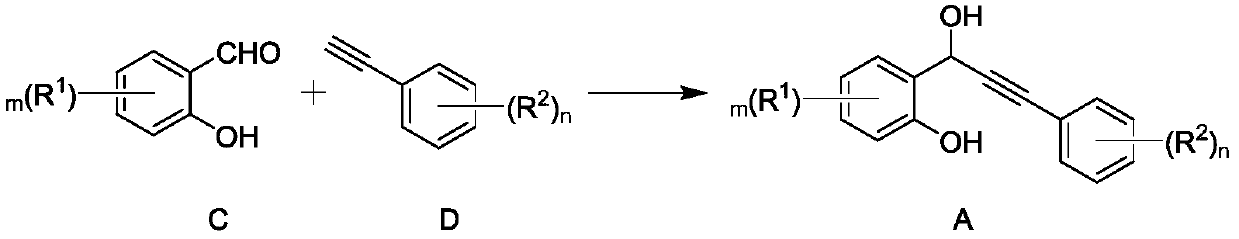
[0028] Specifically, the synthesis method of the present invention is shown in the following formula. Under an oxygen atmosphere, the compound represented by formula A is reacted in a reaction system containing catalyst Co-NC to generate flavonoids represented by formula B. Catalyst Co-NC was prepared by the following method: cobalt salt, terephthalic acid, triethylenediamine were dissolved in DMF (N,N-dimethylformamide), and g-C was added under stirring 3 N 4 (Graphite phase carbon nitride), remove the solvent after the reaction, heat the solid to 850-950°C under an inert atmosphere, preferably to 900°C for 1 hour, and obtain the catalyst Co-NC after cooling. g-C 3 N 4 As a polymer semiconductor, it can be prepar...
Embodiment 1
[0055]
[0056] Under a nitrogen atmosphere, 2.79 mL (22 mmol) of p-methylphenylacetylene, 8.4 mL (2.5 M) of butyllithium n-hexane solution and 30 mL of dry tetrahydrofuran were added to a Schlenk tube, and the mixture was stirred at -78°C for 4 h. 1.91 g (10 mmol) of 3,5-dichlorosalicylaldehyde was added thereto, and stirring was continued at -78°C for 12 h. After the system was cooled to room temperature, 30 mL of saturated ammonium chloride aqueous solution was added to quench the reaction. The mixture was washed with water (3×20 mL), and the resulting aqueous solution was extracted with ether (3×20 mL). Combine the organic phase and ether extract, add 2 g of anhydrous sodium sulfate for drying, and distill off the organic solvent under reduced pressure. The obtained solid residue was separated by column chromatography (petroleum ether: ethyl acetate = 10:1) to obtain 2.46 g of white solid (A-1), with a yield of 80%.
Embodiment 2
[0058]
[0059] Under an oxygen atmosphere, in a sealed tube of 100 mL, add 307.2 mg of substrate A-1 (1 mmol), 15.6 mg of 2,2'-bipyridine (10 mol%), 400 mg of catalyst Co-NC, 15 mL of tetrahydrofuran, and light at room temperature Reaction 12h. After the reaction was finished, the catalyst was left to settle, and the supernatant was transferred into a single-necked round-bottomed flask, and 3×5 mL of dichloromethane was added to wash the catalyst, and the washing liquid was combined into a round-bottomed flask, concentrated under reduced pressure, and subjected to column Chromatographic separation (petroleum ether: ethyl acetate = 10:3) gave 280.8 mg of solid (B-1), with a reaction yield of 92%. The characterization data of solid B-1 are as follows:
[0060] 1 H NMR (CDCl 3 ,300MHz): 8.08(d,J=1.9Hz,1H),7.86(d,J=8.2Hz,2H),7.72(d,J=1.9Hz,1H),7.34(d,J=6.1Hz,2H ), 6.82(s,1H), 2.45(s,3H).
[0061] 13 C NMR (CDCl 3 ,75MHz): 176.5, 163.7, 150.5, 143.0, 133.7, 130.8, 130.0,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com