Heteroaryl estrogen receptor regulating agent and application thereof
A technology of estrogen receptors and modulators, applied in the field of medicine, can solve problems such as hot flashes and endometrial cancer, and achieve the effects of promoting regeneration or repair, prolonging shelf life, and enhancing sustained release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] According to the above scheme, the compound A with general formula (I) can be prepared, and its specific preparation steps are as follows:
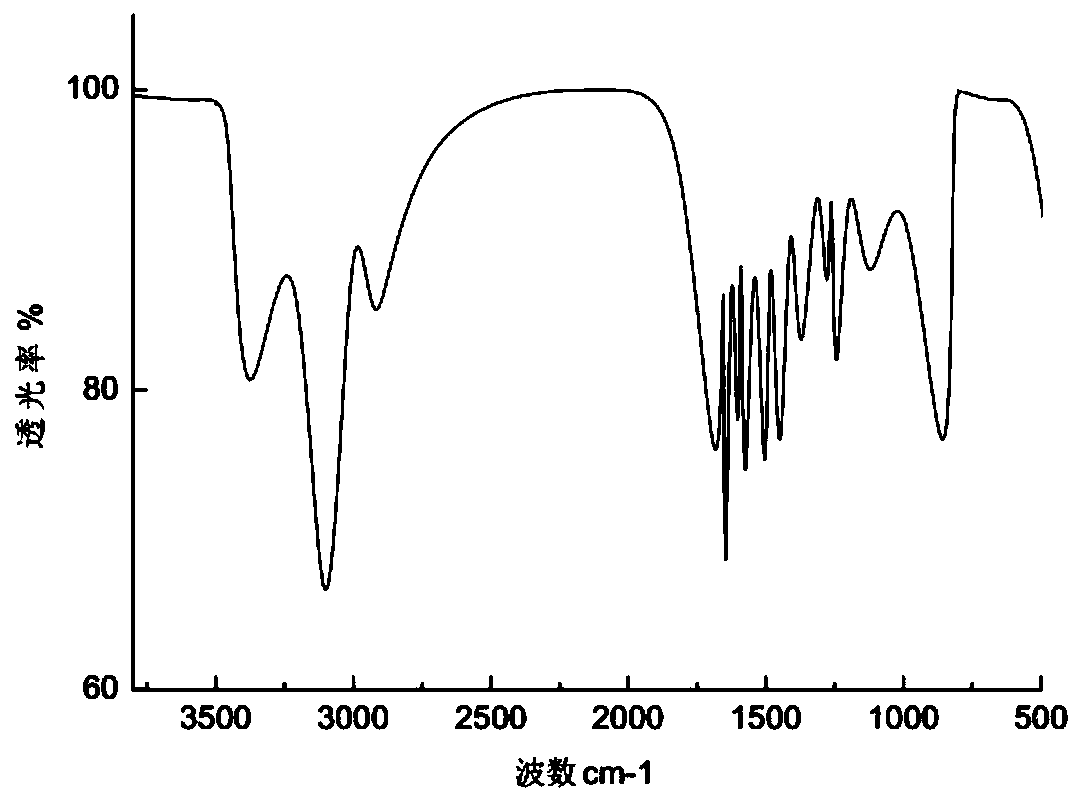
[0072]1) Preparation of intermediate 1: Take 20 g of 4-hydroxyacetone and 100 g of anhydrous potassium carbonate and dissolve them in 20 times the amount of acetone, then add 22 g of low-level benzyl chloride to it, reflux for 24 hours, and evaporate the solvent acetone under reduced pressure , the residue was stirred with water, suction filtered and dried, and the obtained white solid was intermediate 1, m.p.101~102℃, ESI-MS: m / z241 ([M+H] + );
[0073] 2) Preparation of intermediate 2: Take 10 g of intermediate 1 and add it into a mixed solvent of 1,4-dioxane and anhydrous methanol, slowly add 7 g of liquid bromine dropwise, react at room temperature until the solution is transparent and clear, add water and stir for 1 h, Suction filtration and drying, the obtained white solid is Intermediate 2, m.p.74~75℃, ESI-MS: m / z 320 ([M+H...
Embodiment 2
[0084] According to the above scheme, the reddish-brown solid compound B with general formula (I) can be prepared, and the structural formula is as follows:
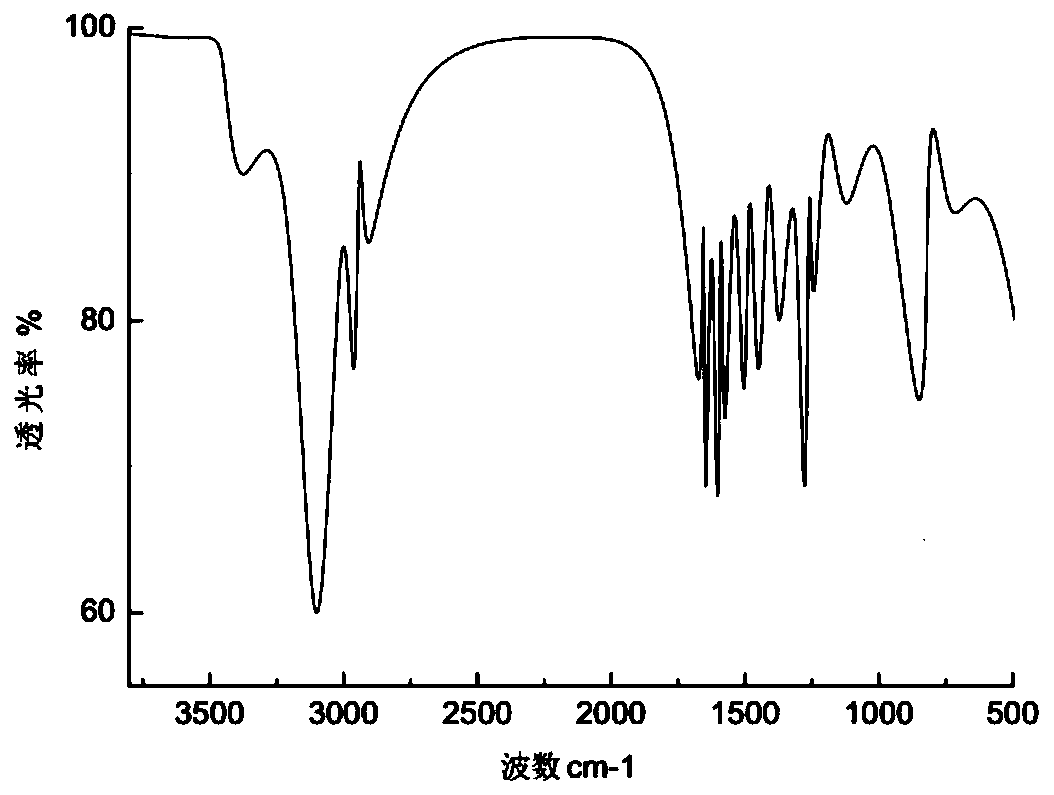
[0085] m.p.107~108℃, ESI-MS: m / z 560([M+H]+), 1H NMR(400MHz, CDCl3): δ7.48(d, J=7.6Hz, 1H), 7.22-7.16(m ,1H),7.13-7.06(m,1H),6.92(d,J=10.5Hz,2H),5.96(s,1H),5.51(s,1H),5.88(d,J=5.0Hz,1H) ,4.53(t,J=5.7Hz,1H),4.41(t,J=6.0Hz,1H),4.27(dd,J=4.0,11.3Hz,1H),4.14-4.07(m,1H),3.84- 3.80(m,1H,3.63(t,J=12.5Hz,2H),3.33-3.18(m,6H),2.93-2.78(m,2H),2.65-2.56(m,4H),1.75-1.67(m ,2H), 1.21-1.19(m,3H). Its infrared spectrum is shown in the attached figure 2 .
Embodiment 3
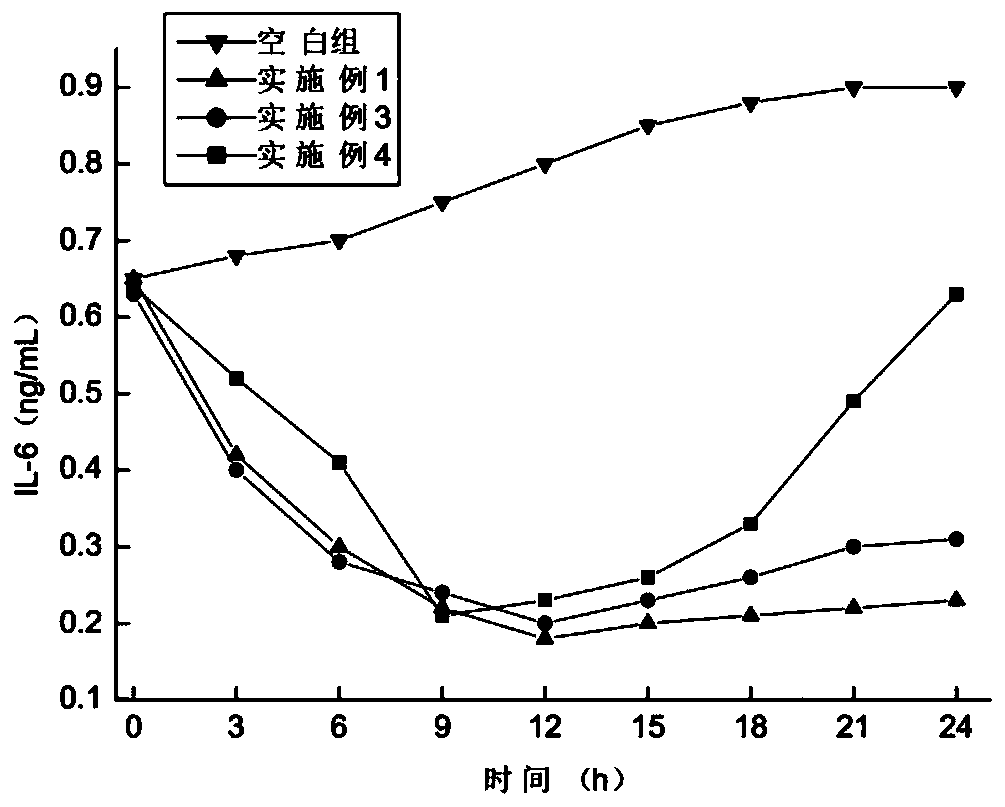
[0087] The preparation method of heteroaryl estrogen receptor modulator, its specific steps are as follows:
[0088] 1) Add alginate to sterile water containing 6 times the amount of propylene glycol, stir to form a uniformly mixed suspension, remove air bubbles to obtain a protective colloid, and the weight ratio of alginate to propylene glycol is 1:3.5;
[0089] 2) adding compound A into the protective colloid, stirring evenly to form a dispersion;
[0090] 3) Dissolve dodecylamine in an ethanol solution with a volume concentration of 75%, then add tetraethyl orthosilicate, calcium nitrate tetrahydrate and triethyl phosphate, and form an emulsion in a water bath for 120 minutes, then age for 18 hours, and wash the obtained Precipitate, freeze-dry the precipitate, and calcinate to obtain the microsphere carrier. The addition amount of tetraethyl orthosilicate is 2.5 times the weight of dodecylamine, and the addition amount of calcium nitrate tetrahydrate and triethyl phosphat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com