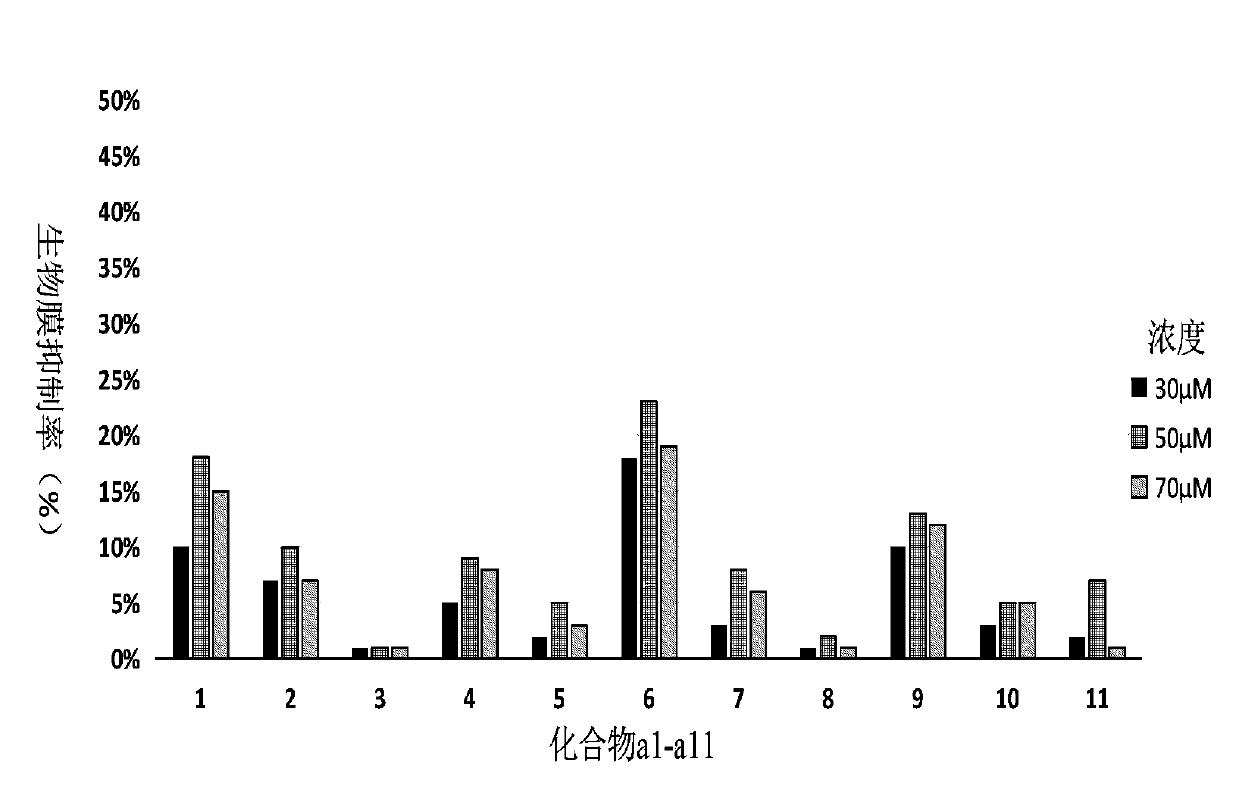
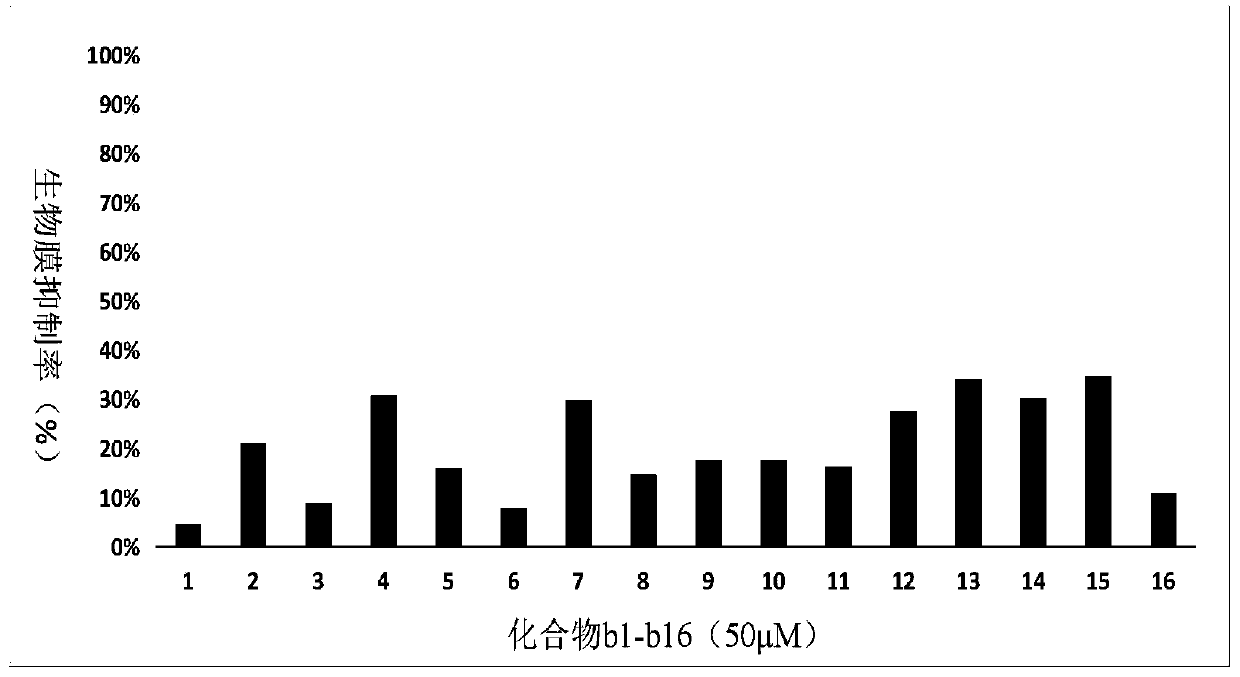
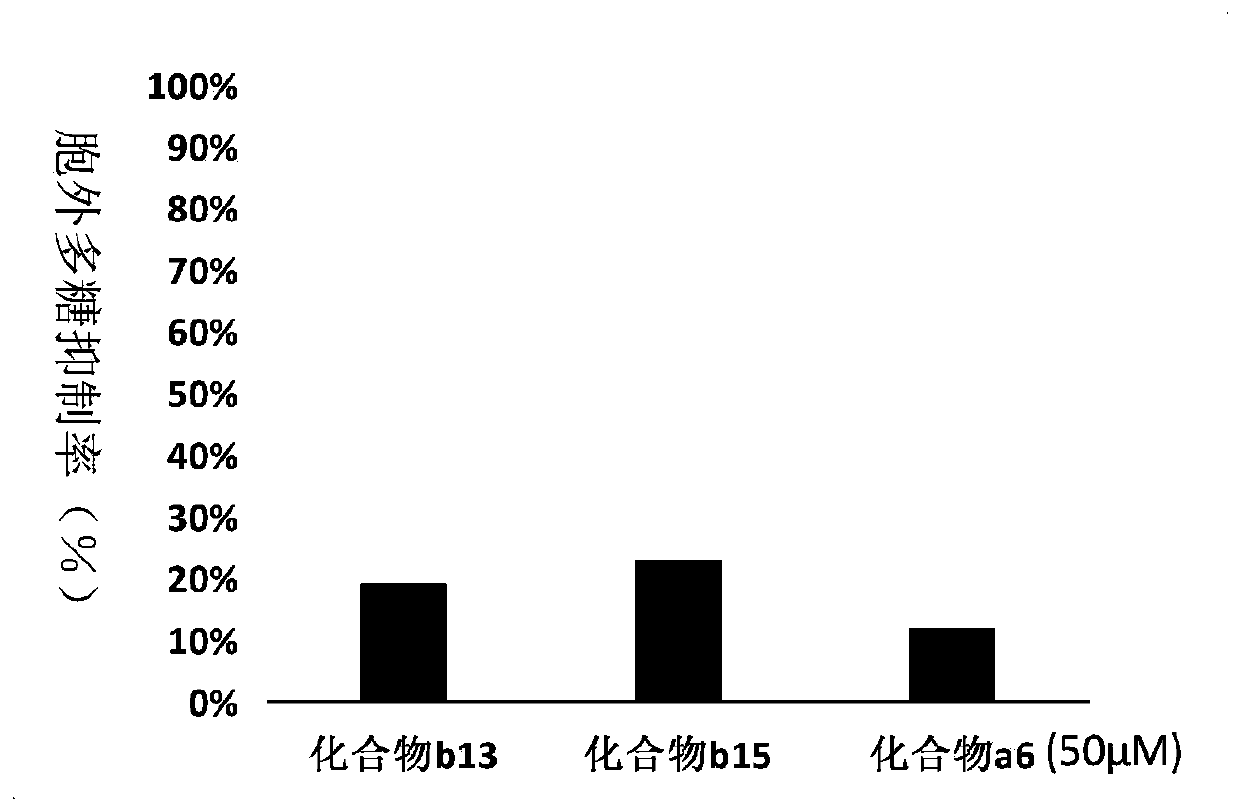
Compound containing cyclohydroxamic acid as well as preparation method and application of same
A technology containing cyclic hydroxamic acid and cyclic hydroxamic acid is applied in the field of biomedicine to achieve the effects of accelerating the reaction rate, reducing the occurrence probability and improving the inhibitory ability of exopolysaccharides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Example 1 Preparation of compound a1.
[0070] A preparation method of compound a1 containing cyclic hydroxamic acid, the reaction equation is as follows:
[0071]
[0072] The concrete reaction steps of above-mentioned reaction equation are as follows:
[0073] A. Add 1.63g of 10mmol isatoic anhydride to 15ml of solvent DMF in a 100ml round-bottomed flask under a nitrogen atmosphere. After dissolving, a mixed solution is obtained. Then put the mixed solution in an ice bath and add 200mg of isatoic anhydride to the round-bottomed flask. 5mmolNaH, then placed at room temperature and stirred for 1h, then added 2ml of 15mmol bromooctane to the mixed solution, stirred at room temperature for 20h, added the ice-water mixture to precipitate a solid, and obtained a filter cake by suction filtration with a Buchner funnel, and then The filter cake is washed with water and petroleum ether, the filtrate is discarded, and the white solid compound c of 2g is obtained after dryin...
Embodiment 2
[0079] Embodiment 2 prepares compound b1,
[0080] A preparation method of compound b1 containing cyclic hydroxamic acid, the reaction equation is as follows:
[0081]
[0082]The concrete reaction steps of above-mentioned reaction equation are as follows:
[0083] A. Add 10mmol of isatoic anhydride to 15ml of solvent DMF in a round-bottomed flask under nitrogen atmosphere, and dissolve to obtain a mixed solution, then add 5mmolNaH to the mixed solution in an ice bath, and then stir at room temperature for 1h , then add 15 mol of brominated raw materials to the mixed solution, stir at room temperature for 10 h, add ice-water mixture to precipitate a solid, filter with a Buchner funnel to obtain a filter cake, then wash the filter cake with water and petroleum ether, discard Filtrate, after drying, obtains the compound e of white solid; In equation (1), DMF is dimethylformamide;
[0084] B, the 2mmol compound e in step A (R2 is-(CH 2 ) 13 CH 3 ), 6mmol hydroxylamine hyd...
Embodiment 3
[0089] Embodiment 3 prepares compound a2,
[0090] The difference from Example 1 is that when R 1 Take-(CH 2 ) 2 CH 3 , Compound a2 is obtained as the final product compound a. The properties and mass spectrum of the product of the structural formula of compound a2 are all tested as follows:
[0091] 3-Hydroxy-2-isopropyl-1-octyl-2,3-dihydroquinazolin-4(1H)-one (compound a2): white solid; yield 88%; m.p.: 75-76℃ ;
[0092] HRMS (ESI-TOF) m / z: Molecular formula is C 19 h 30 N 2 o 2 , detection value [M+Na] + =319.2390, the calculated value is 319.2386;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com