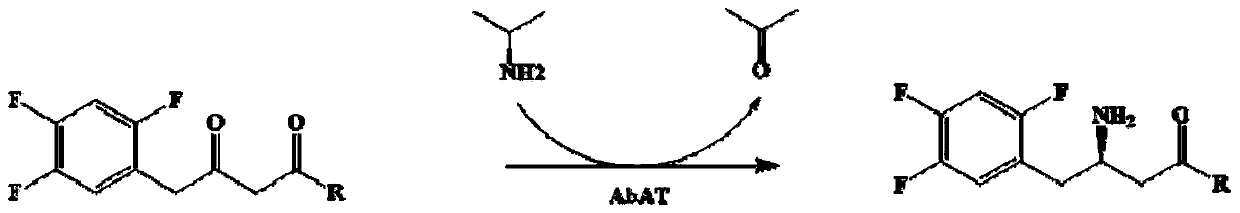
Omega-transaminase mutant and application thereof in preparation of sitagliptin intermediate
A technology of sitagliptin and mutants, applied in the application field of preparing sitagliptin intermediate 1-piperidine-4--1,3-dibutanone, can solve the problem of low conversion rate and low yield 14%, limited application, etc., to achieve the effect of improving the overall conversion rate and shortening the reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Construction and Screening of AbTA Mutants of Transaminase
[0033] 1. Mutant construction
[0034] According to the parental transaminase base sequence from Arthrobacter cumminsii (sequence shown in SEQ ID NO.2, nucleotide sequence shown in SEQ ID NO:1) included in Genbank for homology modeling and molecular In docking, etc., through a large number of calculations and analysis of 336 amino acids (6720 possible mutation types), the 56th and 134th positions were selected to introduce single mutations, the mutation primers for site-directed mutations were designed, and the rapid PCR technology was used to recombine the vector pETDuet / AbTA. As the template, the primers are:
[0035] Primer 1: F56D Pr CGCATTTCCATCGACGACCAGGGCTTTTAT
[0036] F56D Pf AAAGCCCTGGTCGGTCATGGAAATGCGCGC
[0037] Primer 2: F56H Pr CGCATTTCCATCCACGACCAGGGCTTTTAT
[0038] F56H Pf AAAGCCCTGGTCGGTGATGGAAATGCGCGC
[0039] Primer 3: F56V Pr CGCATTTCCATCGTCGACCAGGGCTTTTAT
[0040] F56V Pf AAAGCCCTGGTCGGACAT...
Embodiment 2
[0056] Example 2 Separation and Purification of Transaminase (AbTA)
[0057] The wet bacteria obtained in Example 1 were resuspended in triethanolamine buffer (0.015g / mL, pH 9.0), then ultrasonically disrupted (under ice bath conditions, 70% power for 15min, work for 1s, pause for 1s), 8000rpm After centrifugation for 10 minutes, the supernatant was incubated with the Ni affinity chromatography resin balanced with the above-mentioned binding solution, and then washed with a washing buffer (50mM, pH8.0 sodium phosphate buffer, containing 300mM NaCl, 50mM imidazole) until almost no impurities The protein was then eluted with elution buffer (50mM, pH 8.0 sodium phosphate buffer, containing 300mM NaCl, 500mM imidazole) and the target protein was collected. After the purity was identified by electrophoresis, the target protein was combined and used in dialysis buffer (0.015g / mL, (pH 9.0 ethanolamine buffer) dialysis for 10h (dialysis bag molecular retention 14KD).
[0058] Coomassie br...
Embodiment 3
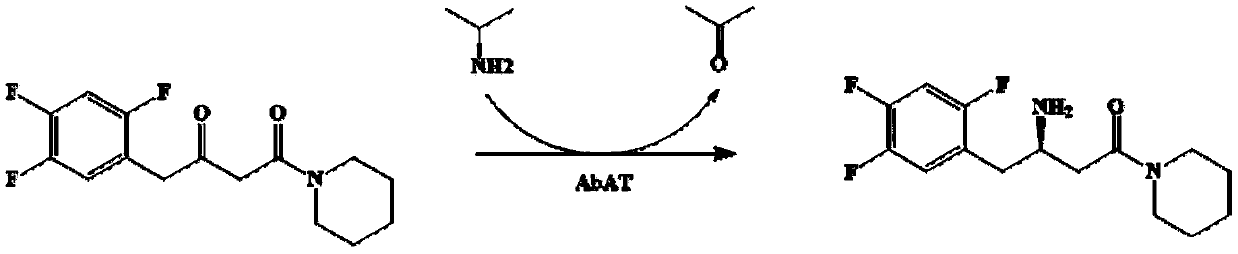
[0059] Example 3 Application of wild-type transaminase AbTA in the preparation of sitagliptin intermediate (R)-3-amino-1-piperidine-4-(2,4,5-trifluorophenyl)-1-butanone
[0060] The recombinant E. coli BL21 / pETDuet-AbTA wet bacteria containing the expression recombinant plasmid obtained by the method of Example 1 or the pure AbTA enzyme obtained by the method of Example 2 was used as a biocatalyst, and sitagliptin intermediate precursor ketone [1- Piperidine-4-(2,4,5-trifluorophenyl)-1,3-dibutyl ketone] is used as the substrate to conduct biocatalytic reaction to synthesize sitagliptin intermediate (R)-3-amino-1 -Piperidine-4-(2,4,5-trifluorophenyl)-1-butanone.
[0061]
[0062] The final concentration composition of the catalytic system (100ml) and the catalytic conditions are as follows: 0.75g of wet bacteria, pH 8-8.5 triethanolamine buffer, substrate sitagliptin precursor ketone 50g / L, final DMSO concentration of 20% (v / v), pyridoxal phosphate 2mmol / L, isopropylamine 80g / L.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com