Detection method of multivitamin injection impurities
A detection method and technology of vitamins, applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of large mutual interference, unfeasible method, and high vitamin content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
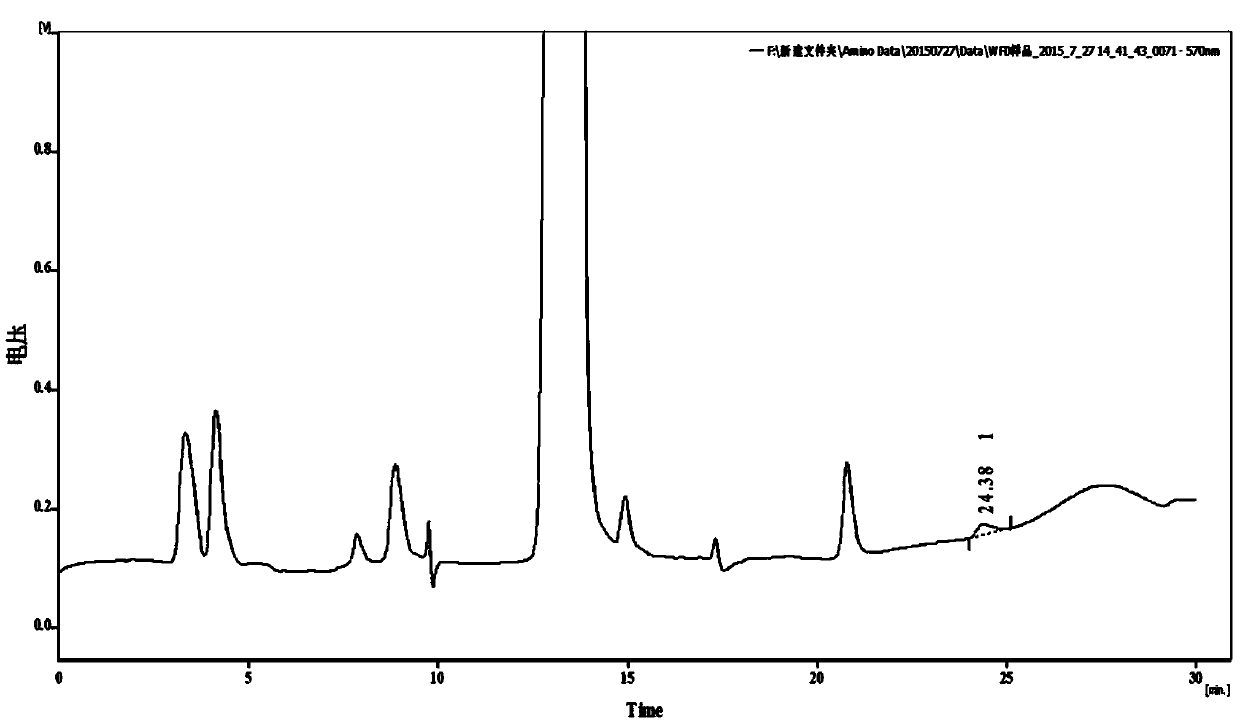
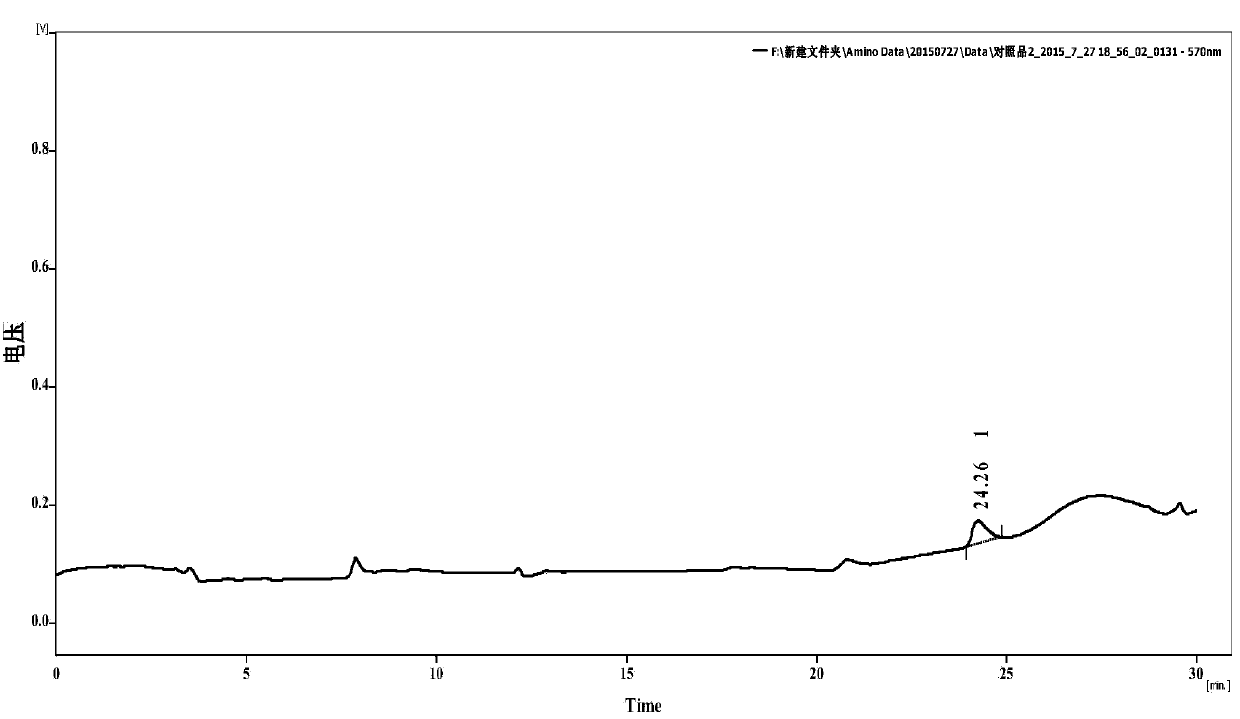
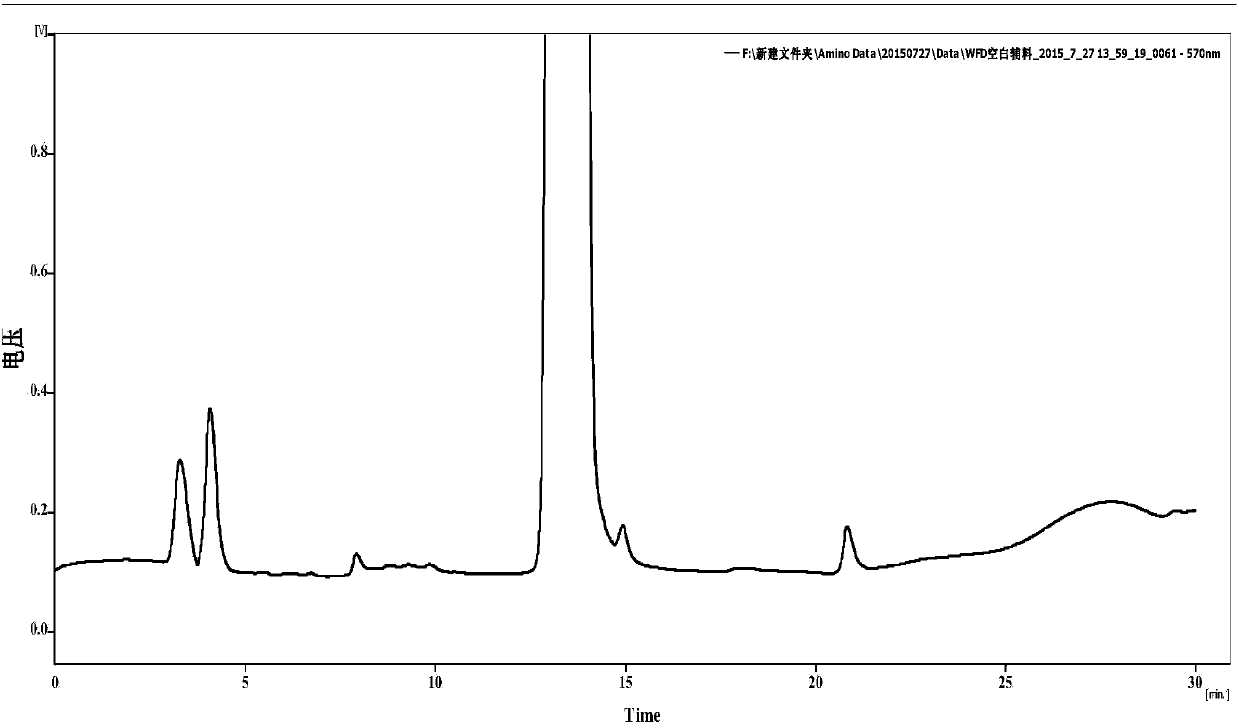
[0097] Oxalic acid detection
[0098] Prescription 1, Prescription 2, Prescription 3, and Prescription 6 Sample water injection: take this product, accurately measure the solution (about 200mg of vitamin C), add 1ml of dilute acetic acid and 0.5ml of calcium chloride test solution, shake well, and let it stand for 1 hour. Add 0.5ml of water, shake well, and use it as the test solution; another precise measure of this product solution (containing about 200 mg of vitamin C), and operate in the same way until it is "placed for 1 hour" before filtering, and precise measure of oxalic acid solution (take an appropriate amount of oxalic acid, Accurately weighed, dissolved in water and diluted to make 0.5ml of a solution containing 1.2mg per 1ml, added to the filtrate, shaken well, left for 1 hour, as a control solution. The turbidity produced by the test solution shall not be thicker than that of the control solution (0.3%).
[0099] Prescription 1, Prescription 2, Prescription 3, P...
Embodiment 2
[0102] Retinol detection
[0103] Prescription 1, Prescription 2, Prescription 3, Prescription 7, Prescription 8, Prescription 9 Sample water injection: Avoid light. Precisely measure the solution of this product (approximately equivalent to vitamin A 6600IU), put it in a 250ml separating funnel, add 40ml of 80% ethanol solution, shake well, add 25ml of n-hexane precisely, shake and extract for 30 minutes, and place it until the upper layer is clear , accurately pipette 10ml of the supernatant and place it in a conical flask, dry it with nitrogen, add 10ml of isopropanol to dissolve it, shake it well, and use it as the test solution; take an appropriate amount of retinol reference substance, accurately weigh Set, add isopropanol to dissolve and quantitatively dilute to make a solution containing about 1.45μg per 1ml, shake well, and use it as the reference solution. Measure according to high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition Sibu general ru...
Embodiment 3
[0109] 3-aminopropanol detection
[0110] Prescription 1, Prescription 2, Prescription 3, Prescription 4 Water injection or freeze-dried powder injection: take a sample, add water to make about 3mg of dexpanthenol per 1ml, as the test solution; take another 3-aminopropanol reference substance Appropriate amount, accurately weighed, dissolved in water and quantitatively diluted to make a solution containing about 30 μg of 3-aminopropanol per 1 ml, as a reference solution. Measured according to high performance liquid chromatography (Chinese Pharmacopoeia 2015 edition Sibu General 0512), using octadecylsilane bonded silica gel as filler (250mm×4.6mm, 5μm); 0.1% heptafluorobutyric anhydride aqueous solution as mobile phase A , with 0.1% heptafluorobutyric anhydride acetonitrile solution as mobile phase B; column temperature is 30 ℃; carry out gradient elution according to the table below; ℃, the carrier gas flow rate is 2.5L per minute). Precisely measure 5 μl, 10 μl, and 20 μl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com